39 electron dot diagram for n2
Bohr Model Questions and Answers - Study.com Bohr Model Questions and Answers. Get help with your Bohr model homework. Access the answers to hundreds of Bohr model questions that are explained in … Draw the Electron-dot Structure of N2 and State the Type ... Draw the Electron-dot Structure of N2 and State the Type of Bonding. - Science. ... Draw the electron-dot structure of N 2 and state the type of bonding. Advertisement Remove all ads. Solution Show Solution. Electron-dot structure of N 2 is. In N 2, one nitrogen atom shares its three electrons with another nitrogen atom to form a covalent bond.
Draw the Lewis dot structures of N2 and CCl4 - Brainly.in 1) Lewis dot structure of N2 is given below: (a) In order to come up with this answer, you first need to know the number of valence electrons for Nitrogen. (b) So N is a member of the Group 5A (based on the periodic table), the number of electrons in its outermost shell must be 5. 2) Lewis dot structure of CCl4 is given below:
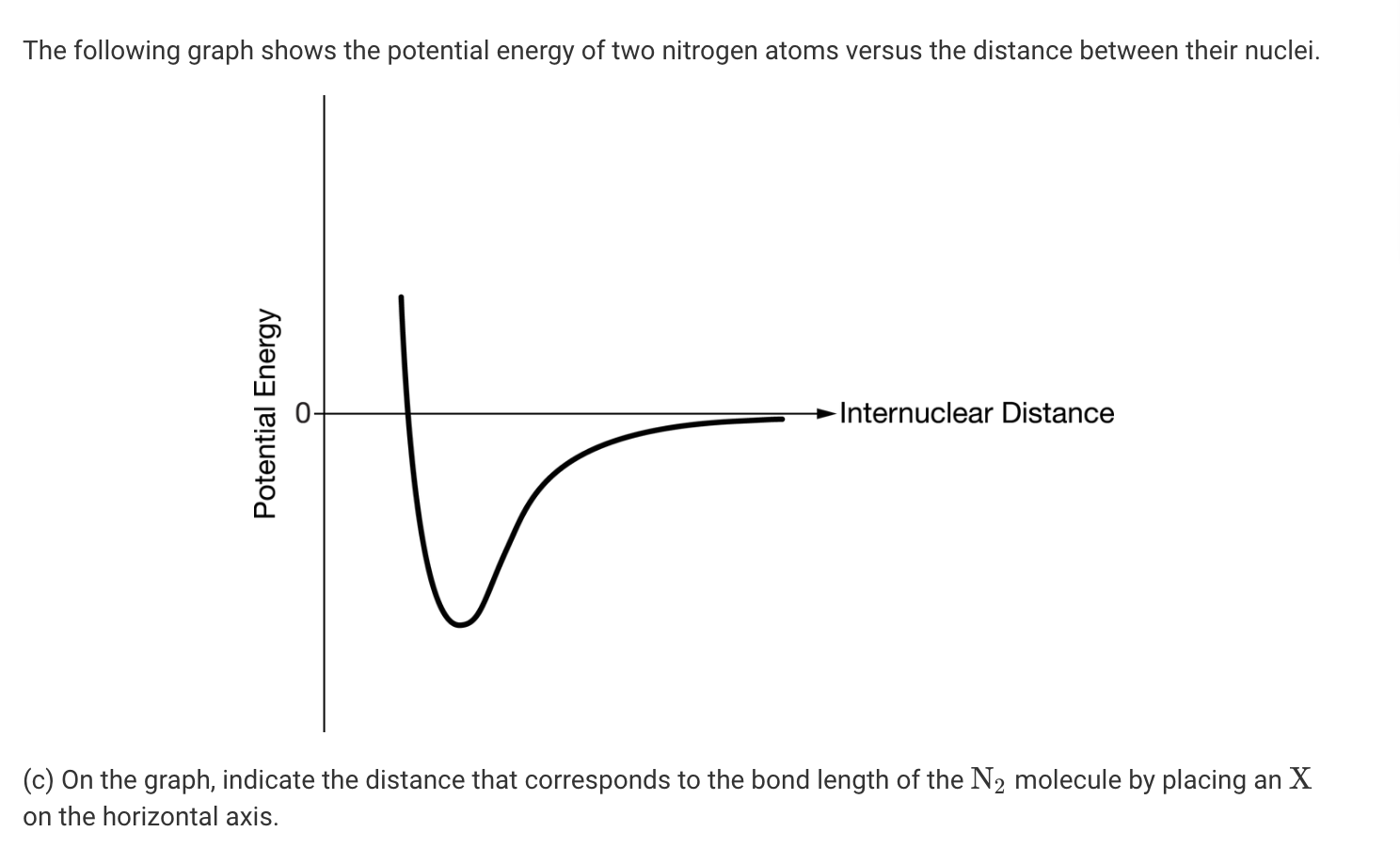
Electron dot diagram for n2
How to determine the Lewis dot diagram for N2 - Quora Originally Answered: How can you determine the Lewis dot structure of N2? So nitrogen has 7 electrons, if we show the electron configuration it's 1S2, 2S2, 2P3, the P sub shell has 3 orbitals with 6 potential electrons and so needs to gain 3 electrons to fill the 2P shell. N2 Lewis Structure | Lewis Structure N2 | HND Assignment The electrons which are bonded with one another are showed by drawing lines, and the excess electrons, which forms the lone pairs are shown with dots. The following is the Lewis Dot Structure for N2, 2N and N2, are basically two forms of the same element. Draw the electron dot structure for oxygen molecule. Click here👆to get an answer to your question ️ Draw the electron dot structure for oxygen molecule. Solve Study Textbooks Guides. Join / Login >> Class 10 >> Chemistry >> Carbon and its compounds >> Covalent bonding in carbon compounds >> Draw the electron dot structure for oxyg. Question .
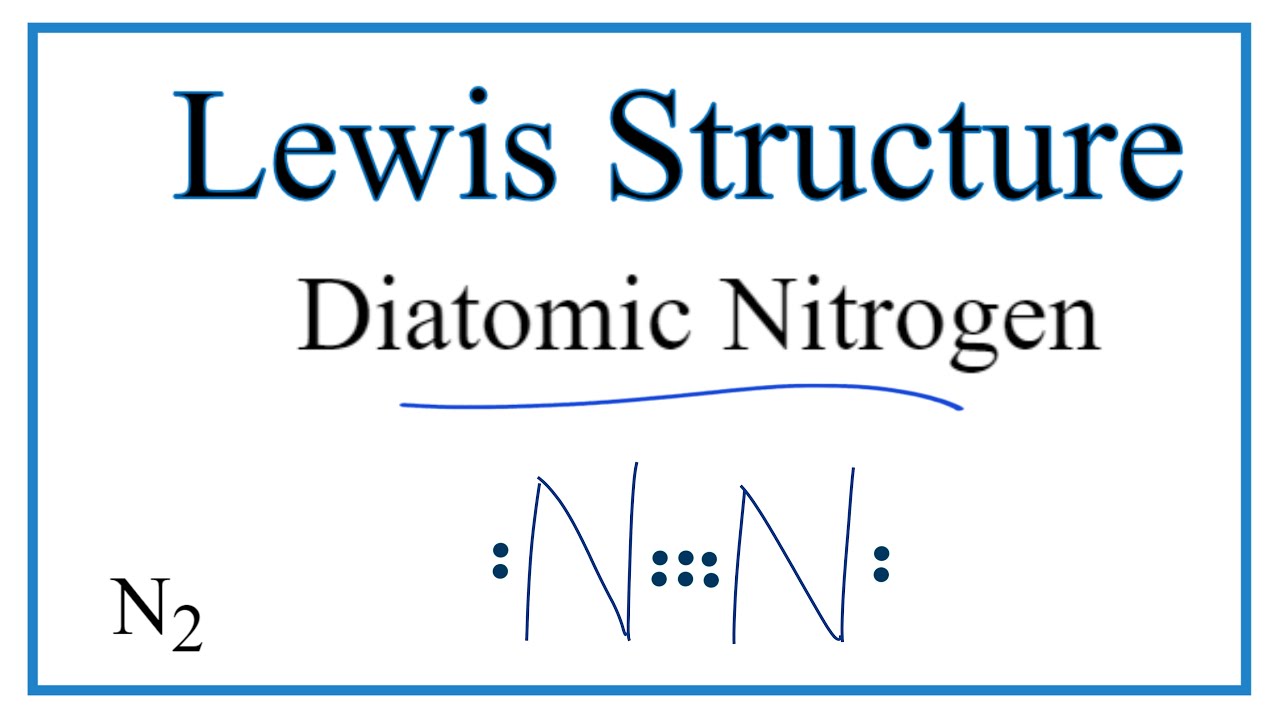
Electron dot diagram for n2. Draw Lewis dot structures of the diatomic molecules 02 and ... Oxygen gas has the molecular formula of O2 and nitrogen gas is N2. The Lewis dot structures that represent both diatomic gases are::O=O: :N:::N: Nitrogen-Doped Carbon Nanodots Produced by Femtosecond ... 17/02/2022 · Understanding the effect of heteroatom doping is crucial for the design of carbon nanodots (CNDs) with enhanced luminescent properties for fluorescence imaging and light-emitting devices. Here, we study the effect and mechanisms of luminescence enhancement through nitrogen doping in nanodots synthesized by the bottom-up route in an intense … Answered: Draw the Lewis Dot Structure for N2.… | bartleby Draw the Lewis Dot Structure for N2. What type of bond keeps this molecule together (single, double, or triple)? Is the bond polar or nonpolar and why? Question. Draw the Lewis Dot Structure for N 2. What type of bond keeps this molecule together (single, double, or triple)? Is the bond polar or nonpolar and why? N2 Lewis Structure: How to Draw the Dot Structure for N2 ... We have two Nitrogens. Multiply those together, we have a total of 10 valence electrons for the N2 Lewis structure. We'll put the two Nitrogens next to each other, and then we'll put two valence electrons between them to form a chemical bond. We have a total of 10 valence electrons. We've used two, go around the outside, 4, 6, 8, and 10.
N2 Lewis Structure - Easy Hard Science N2 Lewis Structure Setup It's easiest to think in terms of dots to make the N 2 Lewis structure. Nitrogen needs to bond three times, shown as the lone dots on the left, right and bottom of the N atoms in the below diagram. There is also a pair of dots, representing two more electrons, that won't bond, on top of each N. Answered: please explain What percentage of a… | bartleby Q: A fuel mixture comprising 600.0g of N204 and 400.0g of CH&N2 is used in a test of laboratory-scale m... A: Given, mass of N2O4=600.0 gmass of CH6N2=400.0 g question_answer Chem quizzes and tests Flashcards | Quizlet label the diagram below with the different types of electromagnetic radiation in the EM spectrum. 1. Gamma rays (10^-12) 2. X-rays (10^-9) 3. Ultraviolet (UV) (10^-8) 4. Visible (10^-7) 5. Infrared (IR) (10^-5) 6. Microwave (10^-2) 7. Radio (10^1.5) Calculate the wavelength of a photon of electromagnetic radiation with a frequency of 1 MHz. 300 m. Give the number of carbon atoms, … Density of States - Engineering LibreTexts Sep 08, 2021 · Figure \(\PageIndex{2}\)\(^{[1]}\) The left hand side shows a two-band diagram and a DOS vs.\(E\) plot for no band overlap. The right hand side shows a two-band diagram and a DOS vs. \(E\) plot for the case when there is a band overlap. In simple metals the DOS can be calculated for most of the energy band, using:
⚗️Based on the Lewis electron-dot diagrams of n2 and n2h4 ... College. answer. answer. answered. Based on the Lewis electron-dot diagrams of n2 and n2h4, compare the length of the nitrogen-to-nitrogen bond in n2 with the length of the nitrogen-to-nitrogen bond in n2h4. 2. N2 Lewis Structure: Full Guide (2022 Updated) The N2 molecule is diatomic, Each Nitrogen atom has one lone pair of electrons. Nitrogen has five valence electrons in the N2 electron dot structure, classified as a group 5 on the periodic table. Align the two Nitrogens and then sandwich two valence electrons between them to make a chemical bond. Draw electron dot structure of CO2,H2O,F2,N2 Click here👆to get an answer to your question ️ Draw electron dot structure of CO2,H2O,F2,N2. Solve Study Textbooks Guides. Join / Login >> Class 11 >> Chemistry >> Chemical Bonding and Molecular Structure >> Basics of Chemical Bonding >> Draw electron dot structure of CO2,H2O,F. Question . Lewis Dot Diagram For N2 - schematron.org There are 3 dots (electrons) in the middle for each Nitrogen atom because Nitrogen molecules form triple. The Lewis Structure for N2 looks easy at first. The problem is that there aren't enough valence electons to give both Nitrogen atoms an octet. You'll need to use . The Lewis Structure for N2 looks easy at first.
Solved In the Lewis electron dot structure for N2, what is ... In the Lewis electron dot structure for N2, what is the total number of additional dots (electrons) shown around each N atom? 2 Question 35 (1 point) Saved In the Lewis electron dot structure for Cl2, hqw many lines (representing bonds) should be shown between the two Cl atoms? 1 A/ Question 36 (1 point) Saved In the Lewis electron dot ...
Lewis Dot Structure of N2 (DiNitrogen) - YouTube I quickly take you through how to draw the Lewis Structure of N2 (DiNitrogen) . I also go over the shape and bond angles.
How to Draw the Lewis Dot Structure for N2: Nitrogen Gas ... A step-by-step explanation of how to draw the N2 Lewis Dot Structure (Nitrogen Gas - Diatomic Nitrogen).For the N2 structure use the periodic table to find t...
Emergence of colloidal quantum-dot light-emitting ... 27/12/2012 · a, Energy band diagram of the first type-IV QD-LED employing ZnO (ref. 4); the electron transport layer of choice in today's high-performance devices. PEDOT, poly(3,4-ethylenedioxythiophene); PVK ...
What is the Lewis dot structure for N2 - Brainly.com Answer: Image result for What is the Lewis dot structure for N2socratic.org. Transcript: For the N2 Lewis structure, we have five valence electrons for Nitrogen--it's in group 5 or 15 on the periodic table. We have two Nitrogens. Multiply those together, we have a total of 10 valence electrons for the N2 Lewis structure.
What is the Lewis structure of N2? - Answers What is the Lewis dot formation of N2? The N2 molecule consists of two nitrogen atoms held together by a triple bond. Each nitrogen atom also has a lone-pair of electrons.
The Oxford Solid State Basics Solutions to ... - Academia.edu Academia.edu is a platform for academics to share research papers.
Draw the Lewis structure for N2. Nitrogen is an unreactive ... The N2 Lewis structure has a triple bond between two nitrogen atoms. According to the octet rule, nitrogen atoms need to bond three times. The N2 molecule is diatomic, meaning that two atoms of the same element are connected in a pair.
Engineering mechanics solved problems pdf - Academia.edu Engineering mechanics solved problems pdf. Provide Data. Download PDF
What Is The Electron Dot Diagram For Nitrogen ... Electron Dot Diagrams lithium 1 s 2 2 s 1 1 valence electron beryllium 1 s 2 2 s 2 2 valence electrons nitrogen 1 s 2 2 s 2 2 p 3 5 valence electrons neon 1 s 2 2 s 2 2 p 6 8 valence electrons. Is N2 dinitrogen or nitrogen? At standard temperature and pressure, two atoms of the element bind to form dinitrogen, a colourless and odorless diatomic ...
PDF Lewis dot structure of n2 molecule Lewis dot structure of n2 molecule Let's take a look at the Lewis of and N2 structure. Atomic nitrogen has 5 valence electrons and 4 orbital valence (2s, 2 px, 2py and 2pcs). In the Lewis structure there is a triple link between nitrogen atoms and a pair of non-binding electrons on each. This is consistent with the physical properties of N2.
Answered: a) UV Cl2 ??? b) UV Cl2 + ??? B. a | bartleby Q: Question 7 The AG'rxn for the reaction: N2(g) + 02(9) + 2 NO(g) is +173 kJ/mol at 25 °C. What is the... A: The relation between standard Gibbs energy change for standard condition and equilibrium constant is...
N2 Lewis Structure, Molecular Geometry, and Hybridization ... Steps to Draw the Lewis structure of N2 Below is the electron dot structure for a Nitrogen molecule: In the Periodic Table, Nitrogen is placed in Group 5 across Period 2. Thus, as per the electronic configuration of the element i.e. 2,5, it has five electrons in its outermost valence shell. As per the molecule N2, it has two atoms of Nitrogen.
Based on the Lewis electron-dot diagrams of N2 and N2H4 ... Based on the Lewis electron-dot diagrams of N2 and N2H4 , compare the length of the nitrogen-to-nitrogen bond in N2 with the length of the nitrogen-to-nitrogen bond in N2H4 1 See answer Advertisement Advertisement Aabdbuyaraba is waiting for your help. Add your answer and earn points.
What is the Lewis structure of N2? | Socratic In order to come up with this answer, you first need to know the number of valence electrons for Nitrogen. Since #N# is a member of the Group 5A (based on the periodic table), the number of electrons in its outermost shell must be 5.. Here is the electron dot structure for a single #N# atom:. The total number of valence electrons between the two #N# atoms is #10 e^-#.
Biology Question Bank For Highschool | Heat Capacity ... The most important characteristic of electron in the production of X-rays is (a) charge of electron (b) mass of electron (c) revolution of electron around the nucleus (d) speed of electron 44. 450 (a) (c) The energy of a photon of light of wavelength nm is 4.4 x 10"' 9 J (b) 2.5 x l o M 9 J 17 K25 x 10~ J (d) 2.5 x 10-'7 J 45. A positron has the same mass as (a) proton (b) a-particle (c ...
Write the electron dot structure for the following ... The electron dot structure of O2 molecule is as shown in the figure below. The electron dot structure of N2 molecule is as shown in the figure below. Was this answer helpful? 1.5 (1) (5) (0) Choose An Option That Best Describes Your Problem. Answer not in Detail. Incomplete Answer.
Answered: a) UV Cl2 ??? b) UV Cl2 + ??? B. a | bartleby Q: Question 7 The AG'rxn for the reaction: N2(g) + 02(9) + 2 NO(g) is +173 kJ/mol at 25 °C. What is the... A: The relation between standard Gibbs energy change for standard condition and equilibrium constant is...
NH2 Lewis Structure, Molecular Geometry, Hybridization ... 25/03/2022 · Lewis Structure is a 2D diagram to represent the internal bonding between constituent atoms in a molecule or ion. Here, we have three key concepts to understand before we can proceed to sketch the Lewis Structure of any given molecule, in this case, azanide ion. 1. Valence electrons and electron dot notations: We already know what valency is ...
Electron Dot Diagrams | Chemistry for Non-Majors Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element's symbol. A beryllium atom, with two valence electrons, would have the electron dot diagram below. Since electrons repel each other, the dots for a given atom are distributed evenly around the symbol before they are ...
17. Acronyms and Abbreviations - NASA 17. Acronyms and Abbreviations 733 BLS. . . . . . . . . .Bureau.of.Labor.Statistics BMI. . . . . . . . . .Bismaleimide BOGS. . . . . . . .Blade.Outer.Gas.Seal
Nitrogen (N2) Molecule Lewis Structure Nitrogen (N 2) Molecule Lewis Structure. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. Lewis structure of N 2 molecule contains a triple bond and each nitrogen atom has one lone pair. There are many things to learn when we draw N 2 lewis structure.. N 2 lewis structure. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom.
Solved 17) The Lewis electron-dot structure of N2 has ... Question: 17) The Lewis electron-dot structure of N2 has _____ nonbonding electrons pairs, bonding electron pairs,_____and a bond order of_____ This problem has been solved! See the answer See the answer See the answer done loading
Answered: Draw the Lewis Dot structure for N2 (on… | bartleby Science Chemistry Q&A Library Draw the Lewis Dot structure for N2 (on paper, not on Canvas) then answer the questions. a. How many total valence electrons are in N2? Express your answer as a whole number. b. How many single bonds are in N2? Express your answer as a whole number.
Draw the electron dot structure for oxygen molecule. Click here👆to get an answer to your question ️ Draw the electron dot structure for oxygen molecule. Solve Study Textbooks Guides. Join / Login >> Class 10 >> Chemistry >> Carbon and its compounds >> Covalent bonding in carbon compounds >> Draw the electron dot structure for oxyg. Question .
N2 Lewis Structure | Lewis Structure N2 | HND Assignment The electrons which are bonded with one another are showed by drawing lines, and the excess electrons, which forms the lone pairs are shown with dots. The following is the Lewis Dot Structure for N2, 2N and N2, are basically two forms of the same element.
How to determine the Lewis dot diagram for N2 - Quora Originally Answered: How can you determine the Lewis dot structure of N2? So nitrogen has 7 electrons, if we show the electron configuration it's 1S2, 2S2, 2P3, the P sub shell has 3 orbitals with 6 potential electrons and so needs to gain 3 electrons to fill the 2P shell.

















![Expert Answer] Draw the electron dot structure of nitrogen ...](https://hi-static.z-dn.net/files/da9/f9dadd9fdaba09b20251ec55355bd804.jpg)


![Expert Answer] Draw the electron dot structure of nitrogen ...](https://hi-static.z-dn.net/files/d2a/14520537102bba9a40f164a746e6ef1e.jpg)


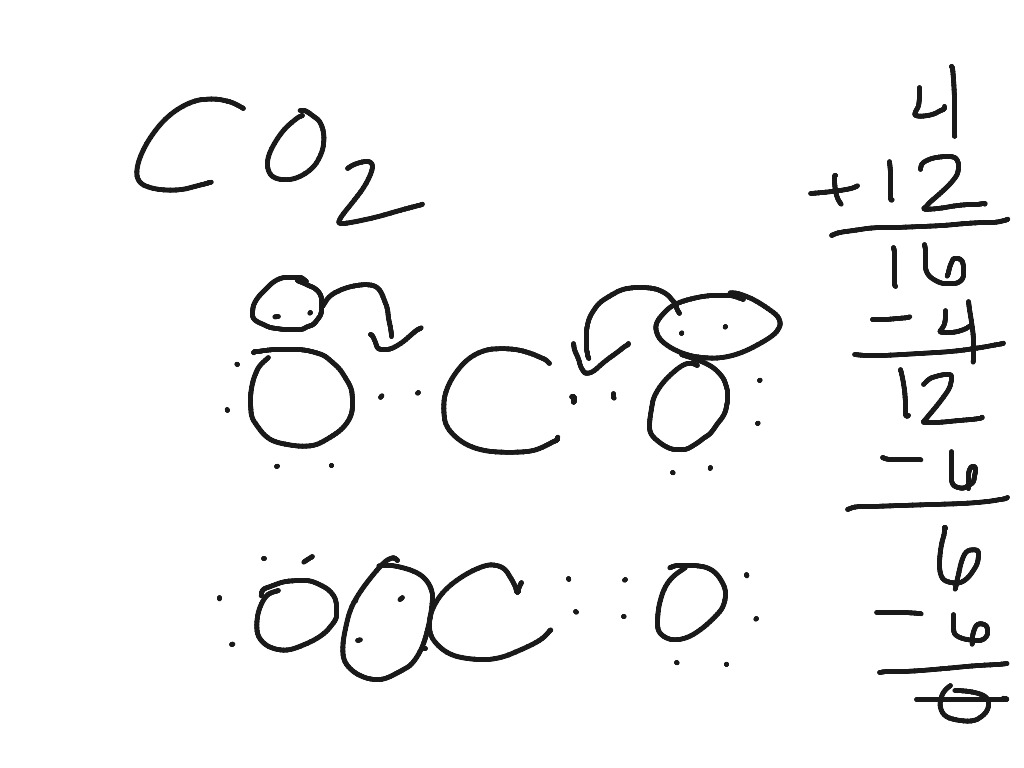



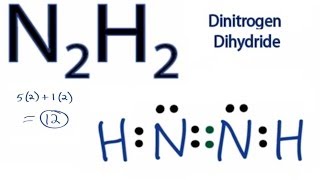

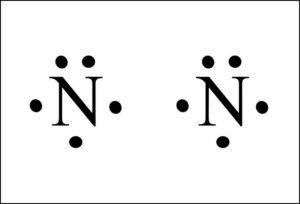

Comments
Post a Comment