39 lewis dot diagram for h+
What is the Lewis dot structure for nitrogen ... What is the dot structure of NO2? The NO2 Lewis structure has a total of 17 valence electrons. It's not common to have an odd number of valence electrons in a Lewis structure. Because of this we'll try to get as close to an octet as we can on the central Nitrogen (N) atom. H2O Lewis Structure - Lewis Dot Structure | Chem Helps Here is the H2O Lewis Structure. We will show how to draw H2O Lewis Dot Structure in this article. Calculate the total valence electrons in the molecule. H 1s1 Valence electron (DE) 1 (2×1= 2 since there are 2 H atoms) O 1s2 2s22p4 Valence electron 6 VE = 6+2 = 8. The number of electrons required for the molecule to obey the octet rule is found (OR).
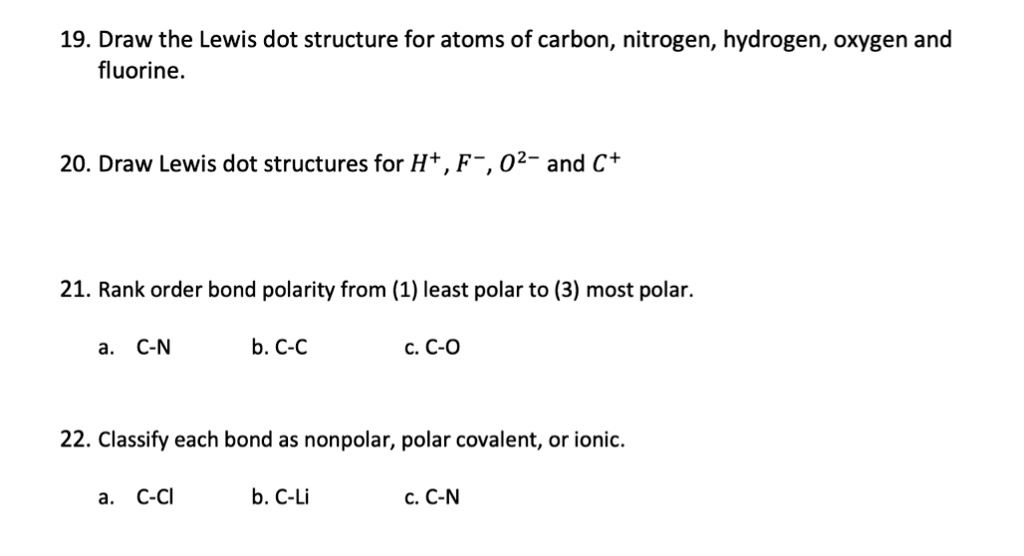
Chemistry Enhanced Scope & Sequence Write the symbol, and fill in the Lewis Dot Diagrams for the 1st four rows and all elements in groups (columns) 1, 2, and 13–18. 3. Use a colored pencil to shade in the two columns where electron configurations end with s1 and s2.

Lewis dot diagram for h+
What is the Lewis dot structure of Na2Co3? 2022 - Question ... That means that it partially dissociates to make H+ and SO4^2-. What is the Lewis symbol of sodium? NaH 5.3: Lewis Diagrams. What is the Lewis symbol for Na+? Na becomes Na+ by losing one electron from its valence shell and getting an overall positive charge. Hence, the Lewis symbol for the sodium ion does not have a dot, but a plus sign as a ... NCERT Xtract - Objective Chemistry.pdf - Chemistry Class ... 21/01/2022 · The orbital diagram in which the Aufbau principle is violated, is :, 2s, 2p, (a), (b), (c), (d), 120. If n = 6, the correct sequence for filling of electrons will be :, (a) ns (n – 2) f (n – 1) d np, (b) ns (n – 1) d (n – 2) f np, (c) ns (n – 2) f np (n – 1) d, (d) ns np (n – 1) d (n – 2) f, 121. Maximum number of electrons in a ... Answered: 5. Methanamide, CH,NO, is a liquid at… | bartleby Explain why in terms of electron domains (VSEPR model). (b) Consider a molecule with the formula CH,O2. The structure of this molecule has a geometry around the carbon atom similar to the geometry around carbon in methanamide. In the box provided below, draw the complete Lewis electron-dot diagram for the molecule.
Lewis dot diagram for h+. What is the lewis dot structure for Fluoride, F1- and Mg2??? What is the Lewis dot structure for the HYDRIDE ion? A)H:- B)H+ C)H' D)H3O+ E)none It's hard for me to read the dots but I THINK answer a) looks like this? H:- If so that is the Lewis dot structure for the hydride ion . Chem. 1) Draw the Lewis structure for CH3NCO, a neutral molecule. 2) Draw the best Lewis structure for NCCH2COCH2CHO, a ... ACELLUS GENERAL CHEMISTRY Flashcards | Quizlet Based on the Lewis/electron dot representation of the two atoms, predict the ratio if metal cationic (+) atom and to nonmetal anionic (-) atom in the compound. ... Which orbital diagram represents fluorine (atomic number = 9) ? ... [H+] for a solution is … Periodic acid | HIO4 - PubChem Periodic acid | HIO4 | CID 65185 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. COVID-19 Information. Public health information (CDC) Research information (NIH) SARS-CoV-2 data (NCBI) Prevention and ... How would you show the bonding of Hydrogen and Oxygen ... Mar 7, 2017 — And thus we have to distribute 8 electrons in the Lewis dot diagram. pic2fly.com. Of course, the electronic geometry is tetrahedral that ...1 answer · There are 2 valence electrons from the hydrogen atoms, and 6 valence electrons from the oxygen atom.......... Explanation: And thus we have to distribute ...
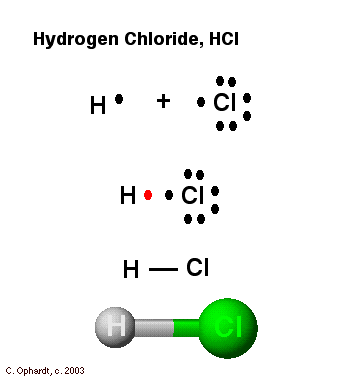
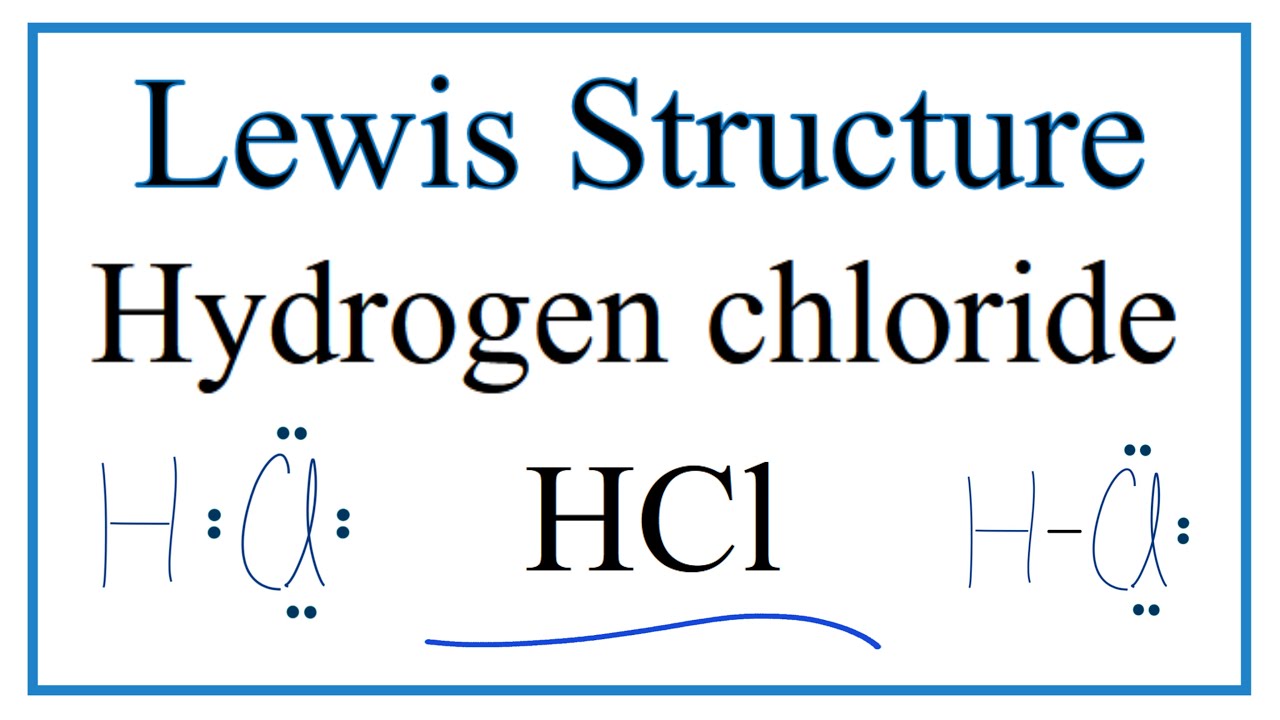
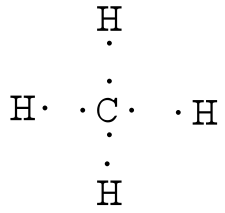
7.3 Lewis Symbols and Structures - Chemistry Calculate the number of valence electrons. HCN: (1 × 1) + (4 × 1) + (5 × 1) = 10H 3 CCH 3: (1 × 3) + (2 × 4) + (1 × 3) = 14HCCH: (1 × 1) + (2 × 4) + (1 × 1) = 10NH 3: (5 × 1) + (3 × 1) = 8. Draw a skeleton and connect the atoms with single bonds. Remember that H is never a central atom: Lewis structure for hydrogen chloride? - Answers The Lewis dot structure for hydrogen chloride starts with a singly bonded H and Cl atom. Around the Cl atom are three pairs of dots. Lewis Electron Dot Diagrams - GitHub Pages The electron dot diagram for helium, with two valence electrons, is as follows: By putting the two electrons together on the same side, we emphasize the fact that these two electrons are both in the 1 s subshell; this is the common convention we will adopt, although there will be exceptions later. Photocatalytic CO2 Reduction: A Review of Ab Initio ... 08/12/2020 · Climate change has prompted scientists to search for possible ways of reducing CO2 emissions or even capturing it from the atmosphere. Catalytic reduction of CO2 into value-added chemicals has been put forward as a viable strategy. While thermocatalytic routes of producing CO, methanol, methane, and higher hydrocarbons from CO2 have been the focus of …
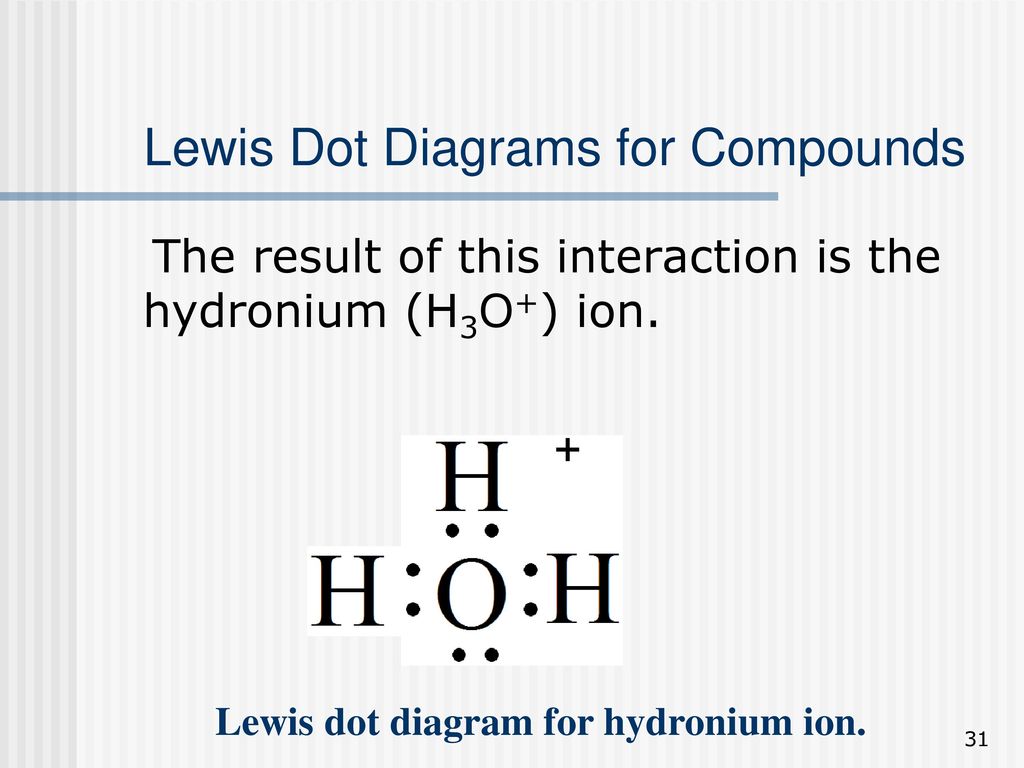
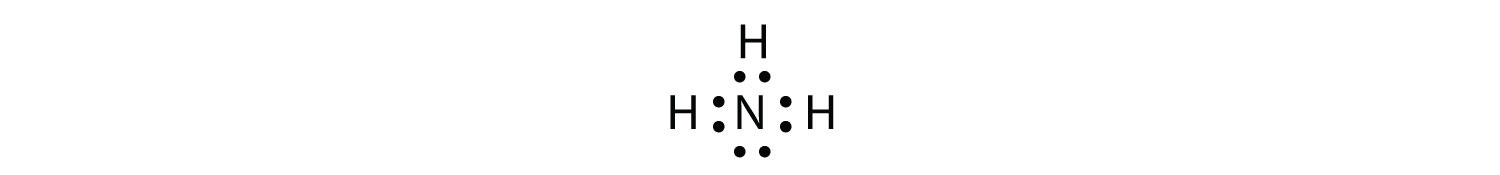
NH4 Lewis Structure - Lewis Dot Structure | Chem Helps NH4 Lewis Structure To write the NH4 Lewis Structure, we need to understand the formation of NH4. Molecules such as NH3, H2O are electron donating ions and molecules. For example, the nitrogen atom in NH3 has an unbonded electron pair. In the H+ ion, there are vacant orbitals that can receive a pair of electrons.… What do the dots in a Lewis dot diagram show and why are ... Lewis dot diagrams use dots arranged around the atomic symbol to represent the electrons in the outermost energy level of an atom. Single bonds are represented by a pair of dots or one line between atoms. ... The lone pair on the nitrogen atom is transferred to the hydrogen ion, making the NH3 a Lewis base while the H+ is a Lewis acid. Lewis Dot Diagram For H+ Cation - schematron.org The Lewis symbol, more commonly "Lewis structure," of H+ is just H The hydrogen has been stripped of its only electron to form the positive ion. Lewis Structures (electron dot diagrams) Lewis Structures of Atoms The gained or lost 1e lost Example Lewis Structure (electron dot diagram) H+ 2+ 3+ 2e lost Li2O Each lithium atom loses one electron to form 2 cations Li+ (2 electrons in. Learning Check: Lewis Dot Structure. Student Study Guide and Solutions Manual ... - Academia.edu Student Study Guide and Solutions Manual for Atkins and Jones's CHEMICAL PRINCIPLES The Quest for Insight FOURTH EDITION
NH4+ Lewis Structure, Molecular Geometry, and ... NH3 + H+ ——> NH4+ Lewis Structure. Lewis Structure is a simplified arrangement and presentation of the electrons present in the valence shell of a molecule. A Lewis Structure is a depiction of the arrangement of electrons in the standalone atoms of an element. In the Lewis Structure, electrons are depicted as dots.
How to Draw the Lewis Dot Structure for H+ (Hydrogen ion ... A step-by-step explanation of how to draw the H+ Lewis Dot Structure.For the H+ structure use the periodic table to find the total number of valence electron...
Lewis Dot Structure and Molecular Shape - eTAP Let's look at one more example of using the Lewis Dot Structure to predict bonding shape and type. Let's do a Lewis Dot structure drawing for Hydrochloric Acid, or HCl. Hydrochloric Acid Lewis Dot Structure. Draw the elemental symbols for Hydrogen and for Chlorine. Draw an H for one Hydrogen and a Cl for one Chlorine.
MakeTheBrainHappy: The Lewis Dot Structure for H2O H2O's Lewis Dot Structure gives it many unique properties mostly due to the two lone pairs on the central oxygen atom. This increases electron-electron repulsion and therefore creates a bent structure as opposed to CO2's linear structure.This "bent" molecular structure gives it many unique properties such as being polar.One of the most fascinating phenomena is the idea of "hydrogen bonding ...
(PDF) Lecture Notes in General and Inorganic Chemistry ... Lecture notes in General and Inorganic Chemistry provides an introduction to the chemistry of inorganic molecules. The emphasis is on basic principles of atomic and molecular structure, thermodynamics, chemical kinetics and catalysis, properties of
What is the Lewis dot structure for N2H2 ... What is the Lewis dot structure for N2H2? In the N2H2 Lewis structure the two Nitrogen (N) atoms go in the center (Hydrogen always goes on the outside). Hydrogen (H) only needs two valence electrons to have a full outer shell. In the Lewis structure for N2H2 there are a total of 12 valence electrons.
What is the Lewis dot structure for the HYDRIDE ion? A)H ... What is the Lewis dot structure for the HYDRIDE ion? A)H:-. B)H+. C)H'. D)H3O+. E)none. It's hard for me to read the dots but I THINK answer a) looks like this? H:-. If so that is the Lewis dot structure for the hydride ion.
NH2- Lewis Structure, Molecular Geometry, Polarity ... It has a total of 8 valence electrons which are participated in the formation of the Lewis dot structure whereas there are 2 bonding pairs and 2 lone pairs of electrons within the molecule. Due to the presence of two lone pairs of electrons that repel bond pairs N-H, it acquires a bent V-shape molecular shape with a bond angle of 104.5 ° .
[Solved] Using the Lewis electron-dot notation, show ... Draw the Lewis electron dot diagram for each ion. a. H+ b. Draw the Lewis electron dot diagram for each ion. a. H+ b. H? Between quarterly audits, a company checks its accounting procedures to detect problems
Introductory Chemistry - Open ... - Open Textbook Library 15/01/2020 · For clarity, Lewis dot symbols should only be assigned to main group elements. The opening essay of chapter 12 (acids and bases) improperly explains the physiological danger of bases as only needing an H+ ion compared to acids needing an OH- ion.
How to Draw the Lewis Dot Structure for IO2 - (Iodite ion ... A step-by-step explanation of how to draw the IO2 - Lewis Dot Structure.For the IO2 - structure use the periodic table to find the total number of valence el...
Solved Select the option below which illustrates the ... Select Lewis dot structure for Catom. A) B) C C C 6 19 21 23 D) C 43 Select Lewis dot structure for H atom. A) H: B) H. C) H+ Select Lewis dot structure for O atom. A) -0 B) -0 C) 0 D) 0 Select the option with the C and O atoms connected to each other. co o.co; Question: Select the option below which illustrates the correct Lewis dot structure ...
Solved Draw the Lewis dot diagram for a H+ cation Make ... Question: Draw the Lewis dot diagram for a H+ cation Make sure charges are in the diagram. This problem has been solved! See the answer See the answer See the answer done loading. Draw the Lewis dot diagram for a H+ cation . Make sure charges are in the diagram. Expert Answer.
PhET: Free online physics, chemistry, biology, earth ... Founded in 2002 by Nobel Laureate Carl Wieman, the PhET Interactive Simulations project at the University of Colorado Boulder creates free interactive math and science simulations. PhET sims are based on extensive education research and engage students through an intuitive, game-like environment where students learn through exploration and discovery.
Lewis Electron Dot Structures - Detailed Explanation with ... Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond.
How to Draw a Lewis Structure - ThoughtCo A Lewis structure is a graphic representation of the electron distribution around atoms. The reason for learning to draw Lewis structures is to predict the number and type of bonds that may be formed around an atom. A Lewis structure also helps to make a prediction about the geometry of a molecule.
What is the Lewis Dot Structure of helium? - Quora Lewis Dot Structure is the structure of an element or molecule, and total valence electrons are as dots to represent bond pairs. In this case "Boron" has a symbol with three boxes next to each other: B for boron; 2s (outer shell) meaning there are two electrons in its highest occupied molecular orbital (Homo Nuclear Force Field), 3 means it also has one electron left over which would then ...
Answered: 5. Methanamide, CH,NO, is a liquid at… | bartleby Explain why in terms of electron domains (VSEPR model). (b) Consider a molecule with the formula CH,O2. The structure of this molecule has a geometry around the carbon atom similar to the geometry around carbon in methanamide. In the box provided below, draw the complete Lewis electron-dot diagram for the molecule.
NCERT Xtract - Objective Chemistry.pdf - Chemistry Class ... 21/01/2022 · The orbital diagram in which the Aufbau principle is violated, is :, 2s, 2p, (a), (b), (c), (d), 120. If n = 6, the correct sequence for filling of electrons will be :, (a) ns (n – 2) f (n – 1) d np, (b) ns (n – 1) d (n – 2) f np, (c) ns (n – 2) f np (n – 1) d, (d) ns np (n – 1) d (n – 2) f, 121. Maximum number of electrons in a ...
What is the Lewis dot structure of Na2Co3? 2022 - Question ... That means that it partially dissociates to make H+ and SO4^2-. What is the Lewis symbol of sodium? NaH 5.3: Lewis Diagrams. What is the Lewis symbol for Na+? Na becomes Na+ by losing one electron from its valence shell and getting an overall positive charge. Hence, the Lewis symbol for the sodium ion does not have a dot, but a plus sign as a ...
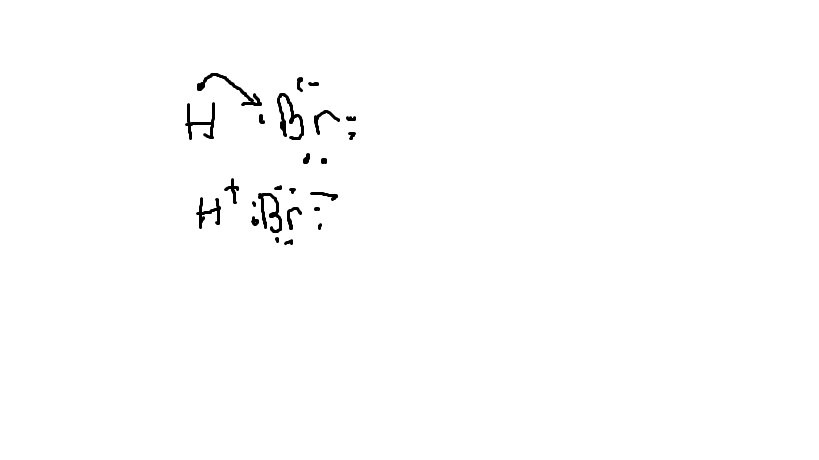
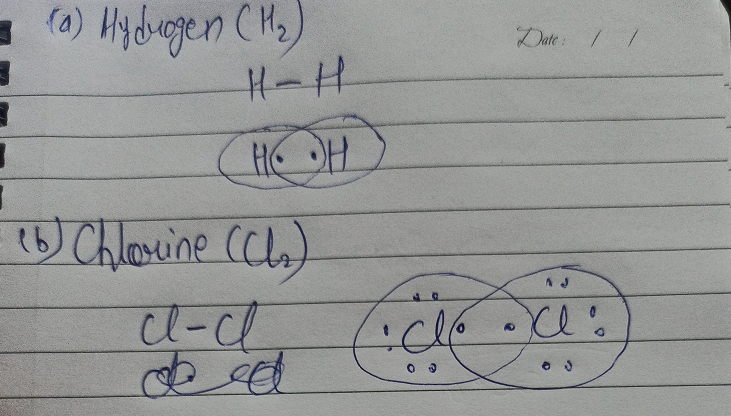










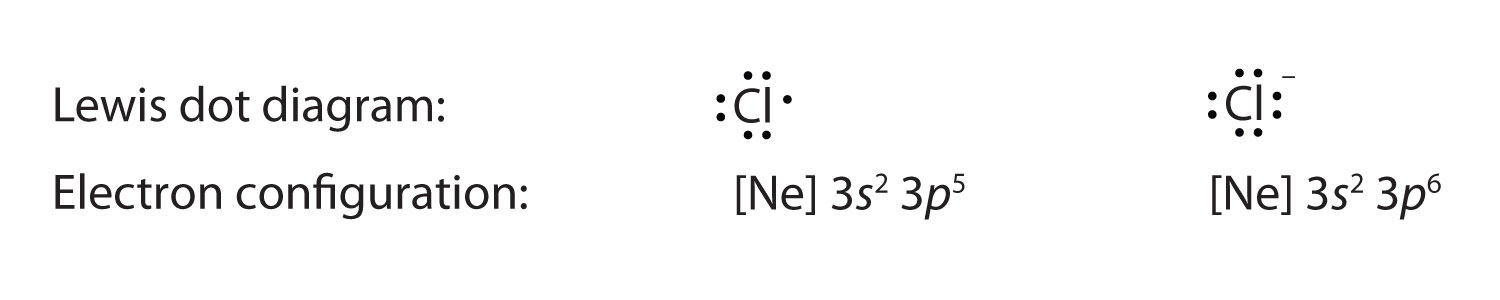










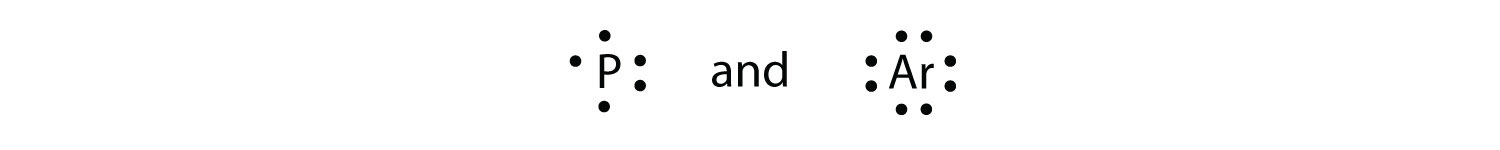
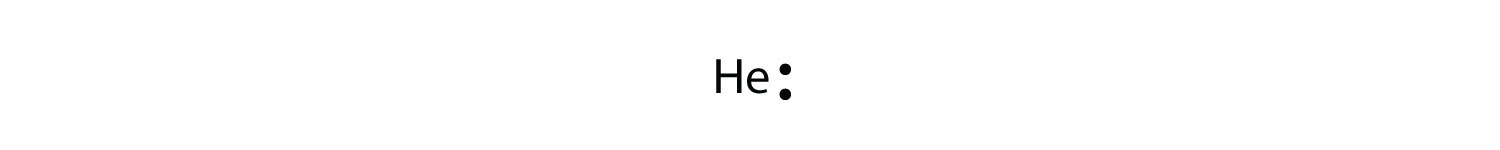

Comments
Post a Comment