42 methane molecular orbital diagram
Ethane. • As molecules get bigger constructing the molecular orbitals. becomes more challenging. • Insights into bonding of larger molecules can be attained by. combining fragments with well defined MO's... through orbital. mixing. • In this manner, ethane can be constructed from MO's of two. pyramidal CH3 groups. A Molecular Orbital Approach to Bonding in Methane methane (CH4) molecule . A molecular orbital diagram showing both the bonding and anti-bonding. It uses 3-D pictorial presentations of molecular orbitals to elucidate organic reaction . As can be seen from the energy diagram - four of the molecular orbitals.
p-orbitals; 3p-orbitals; 3d-orbitals; 4f-orbitals; Compare shape and size of 1s, 2s and 2p orbitals; Molecular Orbitals. Hydrogen; Nitrogen; Fluorine; Ammonia; Methane; Ethylene (Ethene) Acetylene (Ethyne) Allene; Formaldehyde(Methanal) Acrolein; Carbon Monoxide; Hydrogen Fluoride; Allyl Anion; Butadiene; Benzene; Aromaticity of cyclic polyenes ...

Methane molecular orbital diagram
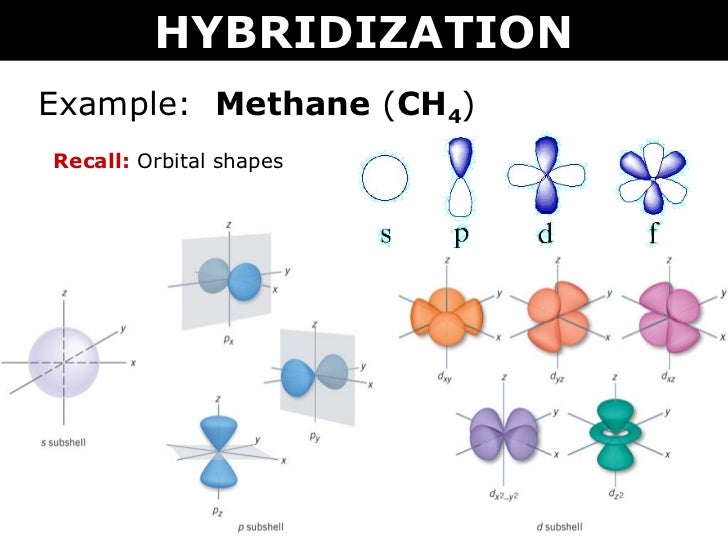
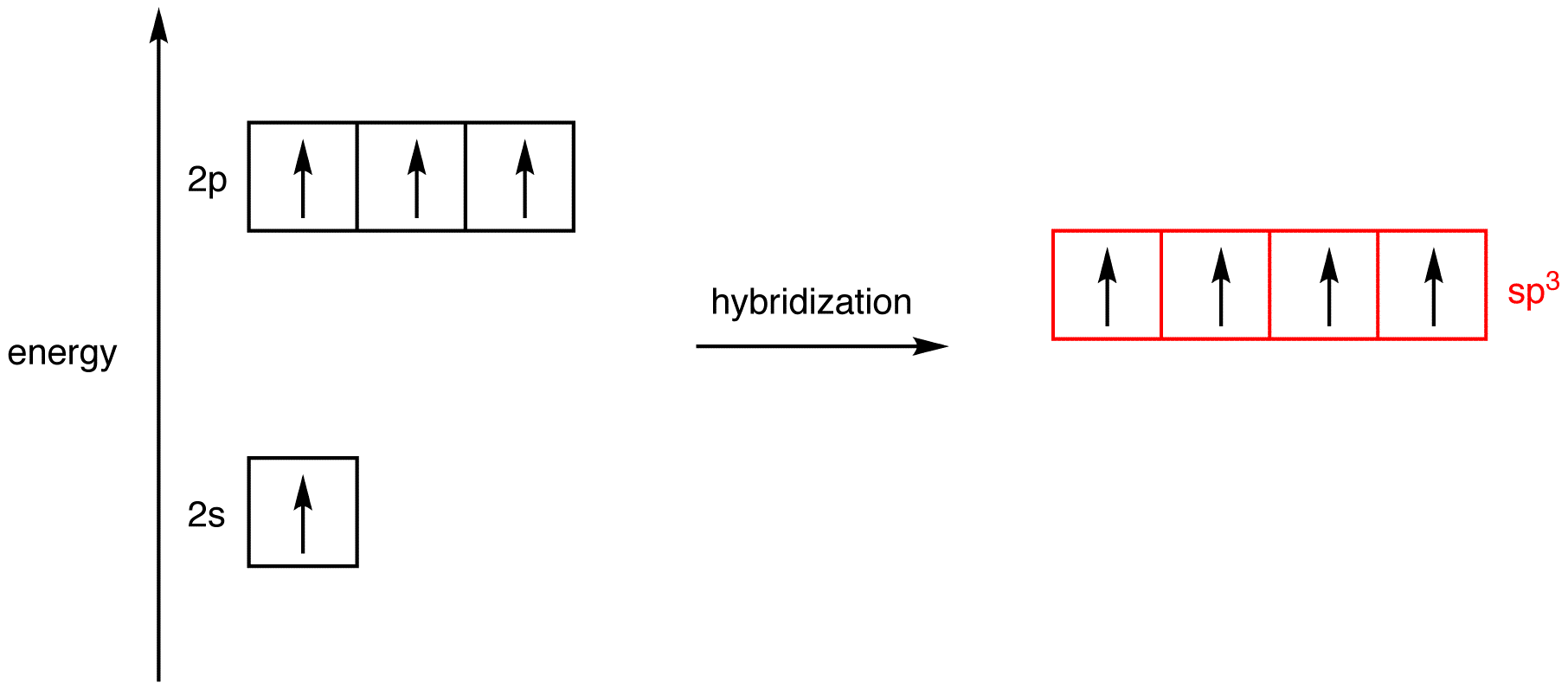
13.08.2021 · Hybridization of s and p Orbitals. In BeH 2, we can generate two equivalent orbitals by combining the 2s orbital of beryllium and any one of the three degenerate 2p orbitals. By taking the sum and the difference of Be 2s and 2p z atomic orbitals, for example, we produce two new orbitals with major and minor lobes oriented along the z-axes, as shown in Figure … square planar methane. With no lone pairs, the electronic and molecular geometry are the same. Technique for constructing LGO's: I Draw Lewis structure and assign VSEPR geometry (already done above) II Assign a point group to the molecular geometry III determine the central atom's VB hybrid orbitals for the electronic geometry Construct the molecular orbital diagram for dichlorine. x y z z y 3 x y z z y 4 Showing the p orbitals. Showing the s and p orbitals. ORBITALS AND MOLECULAR REPRESENTATION 11. CARBON ORBITALS Methane Ethane METHANE AND ETHANE C H H H H CH4 C C H H H H H H C2H6 1 2 Color conventions: Hydrogen atoms are shown in gray.
Methane molecular orbital diagram. 10.05.2017 · Cyclobutadiene: Molecular Orbital Diagram, Antiaromaticity, and Structure. Previously, we’ve seen what the molecular orbitals of benzene look like, and that the fact that they are partially duplexed (or to use the proper nomenclature, “degenerate“) helps to explain benzene’s unusual stability.. Let’s flip the coin. What about cyclobutadiene, a molecule we … NCERT Solutions for Class 11 Chemistry Chapter 4: Chemical Bonding and Molecular Structure “Chemical Bonding and Molecular Structure” is the fourth chapter of the term – I CBSE Class 11 Chemistry Syllabus for session 2021-22. This chapter touches on several fundamental concepts in the field of Chemistry (such as hybridization and the modern theories on chemical … Feb 16, 2017 · How To Draw The Molecular Orbitals of The Allyl Cation, Allyl Radical And Allyl Anion. Drawing the molecular orbitals of a pi system like allyl (3 conjugated p-orbitals) is a bit like construction: build the house (orbitals) first, and fill it with people (electrons) second. Molecular Orbital Diagram for Methane Note that the carbon 1s orbital is omitted from the diagram, since it does not contribute to the bonding. Methane Molecular Orbitals In the following model, the carbon atom is dark gray and the hydrogens are cyan. The hydrogen atoms are arbitrarily numbered.
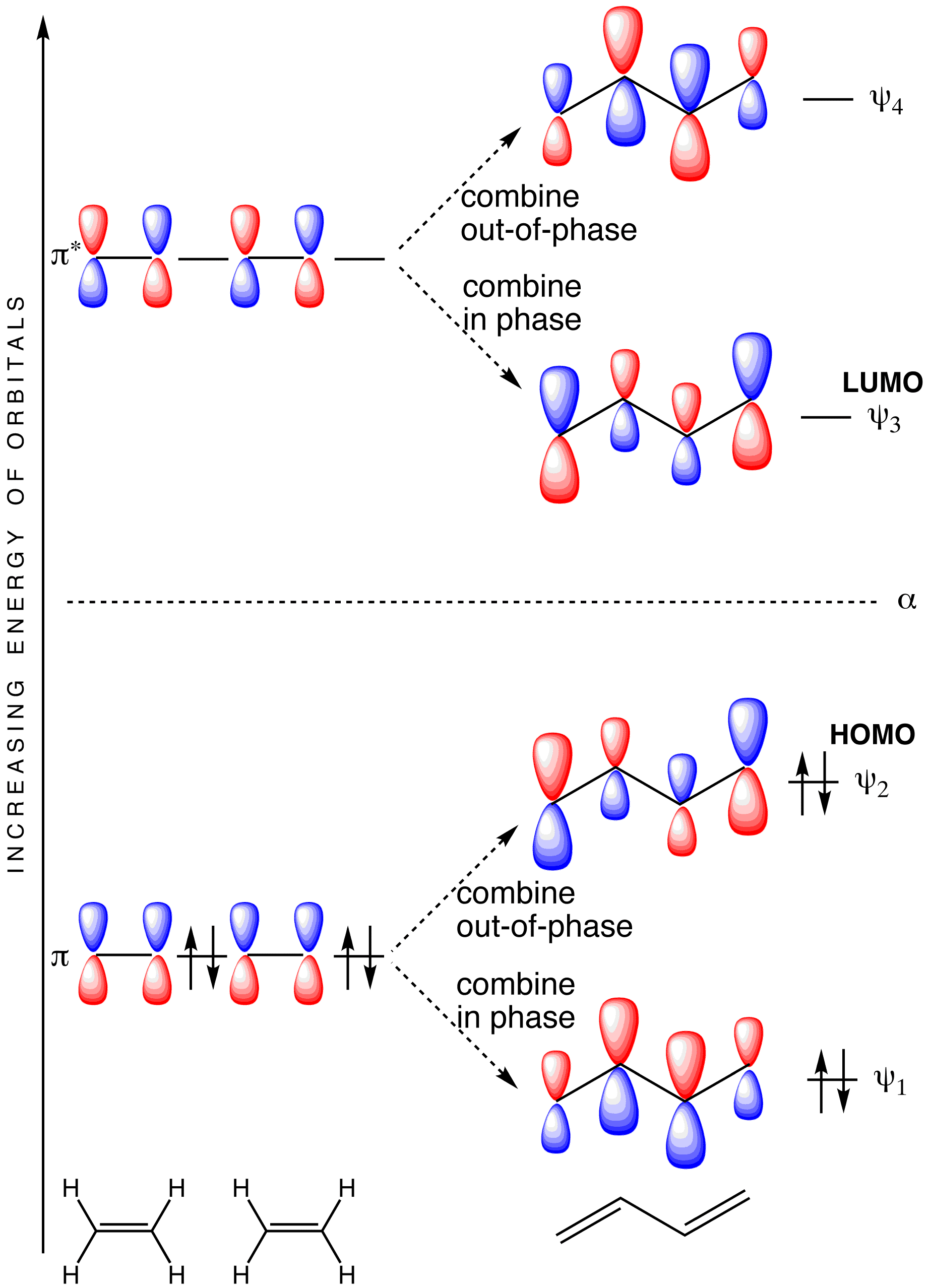
A molecular orbital diagram of ethene is created by combining the twelve atomic orbitals associated with four hydrogen atoms and two sp 2 hybridized carbons to give twelve molecular orbitals. Six of these molecular orbitals (five sigma & one pi-orbital) are bonding, and are occupied by the twelve available valence shell electrons. Milankovitch cycles describe the collective effects of changes in the Earth's movements on its climate over thousands of years. The term is named for Serbian geophysicist and astronomer Milutin Milanković.In the 1920s, he hypothesized that variations in eccentricity, axial tilt, and precession resulted in cyclical variation in the solar radiation reaching the Earth, and that this … Molecular Orbital Theory - Walsh diagram The Walsh diagram shows what happens to the molecular orbitals for a set of molecules which are related in structure. In this case, the difference is the H-X-H bond angle which decreases from 180 o to 90 o Molecular Orbital Theory - Walsh diagram Water 104.5 ° X H H H O H In chemistry, the history of molecular theory traces the origins of the concept or idea of the existence of strong chemical bonds between two or more atoms.. The modern concept of molecules can be traced back towards pre-scientific and Greek philosophers such as Leucippus and Democritus who argued that all the universe is composed of atoms and voids.
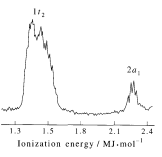
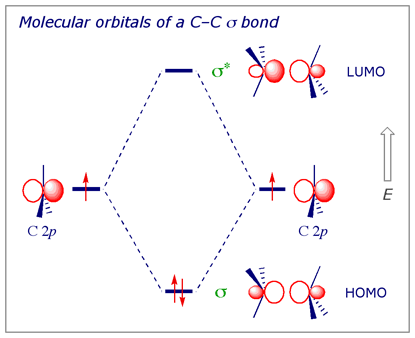
A molecular orbital diagram showing both the bonding and anti‐bonding molecular energy levels is provided below. (McQuarrie & Simon, Physical Chemistry: A Molecular Approach, p. 388) Methane has eight valence electrons, so according to the aufbau and Pauli exclusion principles the two lowest energy molecular orbitals ... Generate the Molecular Orbitals for CH4 (Td), CH4 (D4h) and Cyclopropane using diagram between the bonding MOs of square planar and tetrahedral CH4. The molecular orbital description of bonding in methane does several things for us. Here is an energy level diagram showing how the 4 hydrogen 1s orbitals.Jan 18, · Using LCAO to Construct MOs for ... Aug 14, 2020 · Hybridization of s and p Orbitals. In BeH 2, we can generate two equivalent orbitals by combining the 2s orbital of beryllium and any one of the three degenerate 2p orbitals. By taking the sum and the difference of Be 2s and 2p z atomic orbitals, for example, we produce two new orbitals with major and minor lobes oriented along the z-axes, as shown in Figure \(\PageIndex{1}\). Methane has four valence molecular orbitals (bonding), consisting of one orbital with one nodal plane (lowest occupied) and three degenerate (equal energy) orbitals that do have a nodal plane. For the energy diagram and pictorial view of the orbitals - please see below: Ethane:
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in . A Molecular Orbital Approach to Bonding in Methane methane (CH4) molecule . A molecular orbital diagram showing both the bonding and anti-bonding.
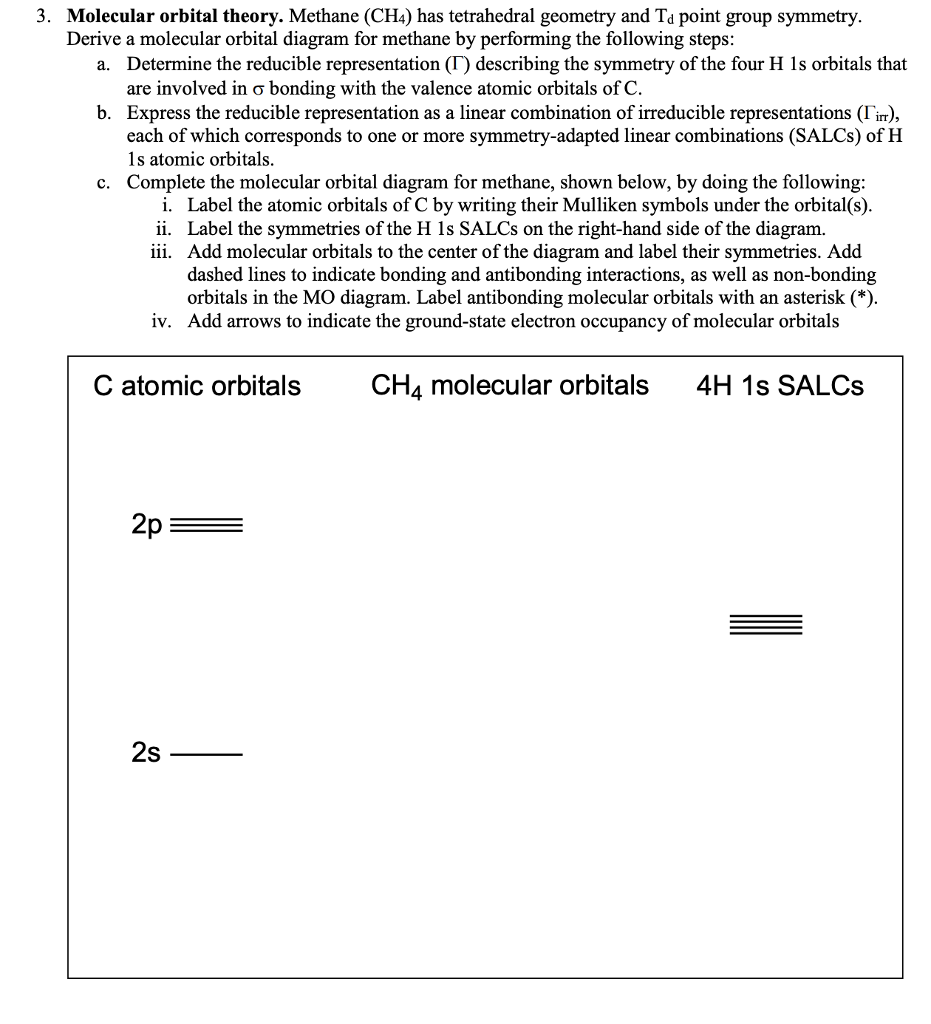
c. Complete the molecular orbital diagram for methane, shown below, by doing the following: i. Label the atomic orbitals of C by writing their Mulliken symbols under the orbital(s). ii. Label the symmetries of the H 1s SALCs on the right-hand side of the diagram. iii. Add molecular orbitals to the center of the diagram and label their symmetries.
1 day ago · The molecular orbital diagram of CO2 is as below. A molecular orbital diagram of any compound gives us an idea about the bonding of the orbitals. It also helps us to find the bond order, bond length, bond strength of the molecule. In the diagram, the left-hand side consists of the atomic orbitals of carbon. Likewise, the left side has AO’s of ...
The molecular orbital description of bonding in methane does several things for us. It should reconcile our valence-bond idea of electrons localized between carbon and hydrogen with the "delocalized" picture typical of the MO approach. It should tell us (quantitatively) about the energies of different electrons.

DNA Genotyping and Sequencing. Technician loads robot for genetic studies of the human papillomavirus (HPV) at the Cancer Genomics Research Laboratory, part of the National Cancer Institute's Division of Cancer Epidemiology and Genetics (DCEG).
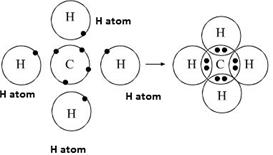
Methane is a pentatomic, tetrahedral molecule. It is formed by combination of one carbon atom with 4 hydrogen atoms. In the molecule of methane, the carbon a...
1 day ago · MO Diagram of CCl4. A MO diagram is nothing but a representation of bonds that are formed within the atoms to form a compound. This diagram is based on Molecular orbital theory. With the help of a MO diagram, the existence of certain compounds can be explained. Here is the pictorial representation of how CCl4’s and CH4 MO diagram looks like.
Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ...
A covalent bond is a chemical bond that involves the sharing of electron pairs between atoms.These electron pairs are known as shared pairs or bonding pairs, and the stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding. [better source needed] For many molecules, the sharing of electrons allows each …
Please explain me the orbital diagram and electron Dot structure of ccl4, h2o, nh3,ch4. why does graphite have high melting or boiling point if their bonding is weak. give one property of hydrogen chloride which agrees with it being a covalent compound. A covalent hydrocarbon molecule having four single covalent bond.
Methane (CH4) lewis dot structure, molecular geometry, electron geometry, polar or nonpolar, bond angle Home > Chemistry Article > CH4 lewis structure and its molecular geometry Methane is a colorless and odorless gas formed from one atom of carbon and four atoms of hydrogen having the chemical formula CH4.
The photo-isomerization shown above is one example of a general family of reactions known as di-π-methane rearrangements, other examples of which are illustrated in the following diagram. These transformations are often photo-sensitized, indicating they proceed by way of triplet excited states. As the name suggests, substrates exhibiting this rearrangement are comprised of two π …
Molecular orbital theory. Methane (CH4) has tetrahedral geometry and Td point group symmetry. Derive a molecular orbital diagram for methane by performing the following steps: a. Determine the reducible representation (Γ) describing the symmetry of the four H 1s orbitals that are involved in σ bonding with the valence atomic orbitals
A molecule is an electrically neutral group of two or more atoms held together by chemical bonds. Molecules are distinguished from ions by their lack of electrical charge.. In quantum physics, organic chemistry, and biochemistry, the distinction from ions is dropped and molecule is often used when referring to polyatomic ions.
Molecular Orbital Analysis of Ethene Dimerisation π Molecular Orbitals of 1,3- Butadiene essentially the same theory about how acids and bases behave. π Molecular Orbitals of Ethene The diagram to the right shows the relative energies of the atomic p orbitals, the resulting π molecular orbitals and the electron.
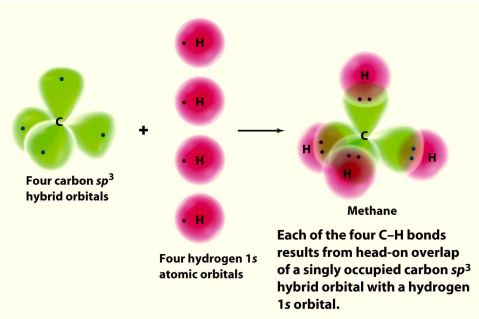
Hybrid Orbitals In order to explain the structure of methane (CH 4), the 2s and three 2p orbitals are converted to four equivalent hybrid atomic orbitals, each having 25% s and 75% p character, and designated sp 3. These hybrid orbitals have a specific orientation, and the four are naturally oriented in a tetrahedral fashion.
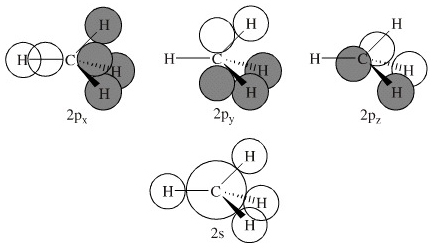
Methane's MOs have a topology similar to the AOs of carbon, but the structure can be very difficult to visualise, so the methane MO construction diagrams A, B and C (below) are shown with the AOs and MOs superimposed upon line structures of the methane.
molecular orbital diagram as a non-bonding molecular orbital. 7. There are a total of 6 electrons to add to the molecular orbital diagram, 3 from boron and 1 from each hydrogen atom. sp Hybrid Orbitals in BeH2 1. The Lewis structure shows that the beryllium in BeH 2 makes 2 bonds and has no lone pairs. It is a linear molecule. Chemistry 104 ...
A molecular orbital diagram showing both the bonding and anti‐bonding molecular energy levels is provided below. (McQuarrie & Simon, Physical Chemistry: A Molecular Approach, p. 388) Methane has eight valence electrons, so according to the aufbau and Pauli exclusion principles the two lowest energy molecular orbitals (2a1 and 1t2) are fully ...

DNA Genotyping and Sequencing. Technician prepares source samples of DNA for quality-control checks during high-throughput genotyping and sequencing at the Cancer Genomics Research Laboratory, part of the National Cancer Institute's Division of Cancer Epidemiology and Genetics (DCEG).
Chapter 2. Alkanes and Cycloalkanes: Introduction to Hydrocarbons 2.1: Classes of Hydrocarbons molecules that are made up of carbon and hydrogen
The three molecular orbitals (MOs) of methane in the ground electronic state (X 1 A 1 ), namely, the core MO, 1 a1 and the valence MOs, i.e. 2 a1 and three-fold energy degenerate MOs 1 t2, are studied in both coordinate space and momentum space.
Now 4 orbital means CH3F has Sp³ hybridization.Carbon and Fluorine has also Sp³ hybridization and Hydrogen atoms are unable to hybridize so leave it as usual. The molecular shape or electron geometry of CH3F is Tetrahedral because three hydrogens and one fluorine atom are bonded to the carbon central atom and no lone pair is present on the central atom that makes …
molecular orbitals. 2.The number of molecular orbitals formed is the same as that of the number of atomic orbitals combined. 3.The additive overlap results in the bonding molecular orbital while the subtractive overlap results in the antibonding overlap. 4.The energy of bonding molecular orbitals is lower than their nonbonding counterparts ...
This in-class activity walks students through the preparation of a molecular-orbital diagram for methane in a square-planar environment. The students generate ligand-group orbitals (LGOs) for the set of 4 H(1s) orbitals and then interact these with carbon, ultimately finding that such a geometry is strongly disfavored because it does not maximize H/C bonding and leaves a lone pair on C.
This animation explains the methane molecule through the Molecular Orbitals TheoryFor more Chemistry animations check out the youtube channel Lili Tosta: htt...
Properties and bonding. Methane is a tetrahedral molecule with four equivalent C-H bonds.Its electronic structure is described by four bonding molecular orbitals (MOs) resulting from the overlap of the valence orbitals on C and H.The lowest-energy MO is the result of the overlap of the 2s orbital on carbon with the in-phase combination of the 1s orbitals on the four hydrogen atoms.
Construct the molecular orbital diagram for dichlorine. x y z z y 3 x y z z y 4 Showing the p orbitals. Showing the s and p orbitals. ORBITALS AND MOLECULAR REPRESENTATION 11. CARBON ORBITALS Methane Ethane METHANE AND ETHANE C H H H H CH4 C C H H H H H H C2H6 1 2 Color conventions: Hydrogen atoms are shown in gray.

DNA Genotyping and Sequencing. A technician reviews data from high-throughput DNA genotyping and sequencing at the Cancer Genomics Research Laboratory, part of the National Cancer Institute's Division of Cancer Epidemiology and Genetics (DCEG).
square planar methane. With no lone pairs, the electronic and molecular geometry are the same. Technique for constructing LGO's: I Draw Lewis structure and assign VSEPR geometry (already done above) II Assign a point group to the molecular geometry III determine the central atom's VB hybrid orbitals for the electronic geometry
13.08.2021 · Hybridization of s and p Orbitals. In BeH 2, we can generate two equivalent orbitals by combining the 2s orbital of beryllium and any one of the three degenerate 2p orbitals. By taking the sum and the difference of Be 2s and 2p z atomic orbitals, for example, we produce two new orbitals with major and minor lobes oriented along the z-axes, as shown in Figure …













Comments
Post a Comment