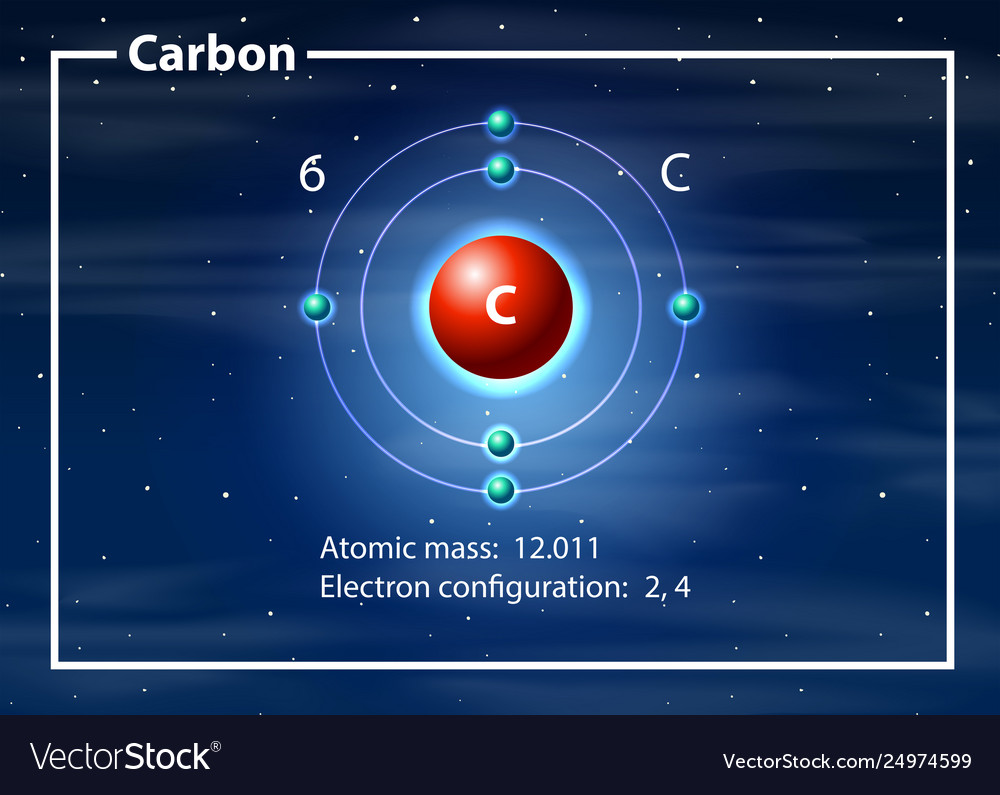
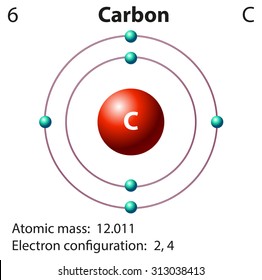
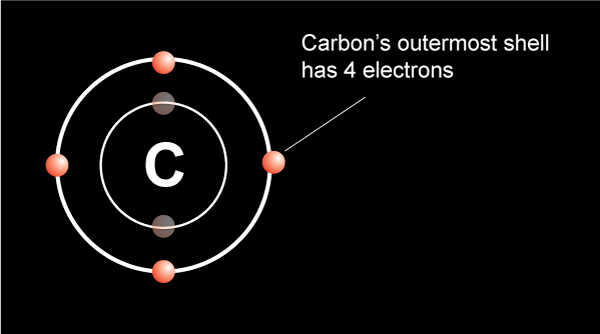
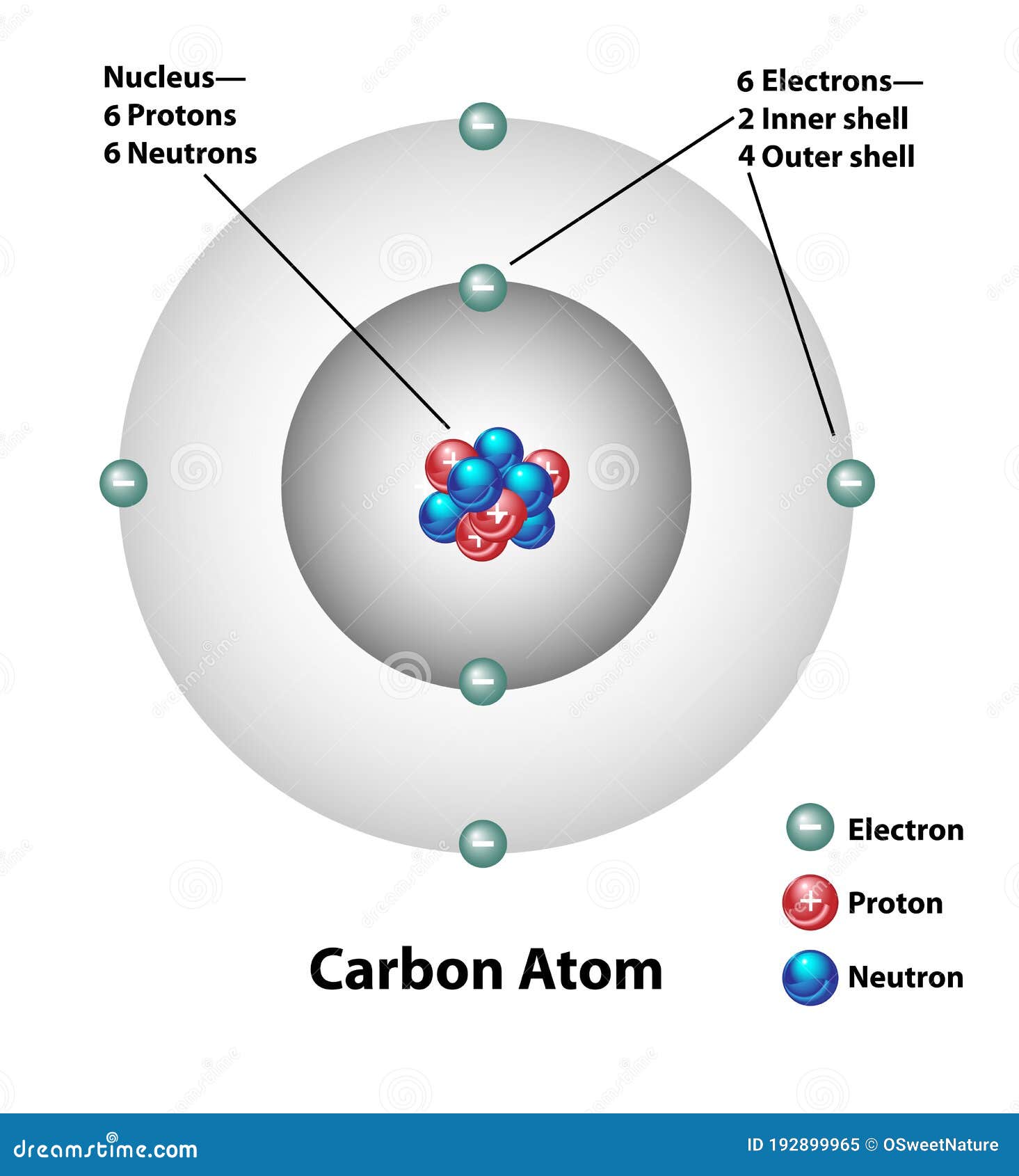
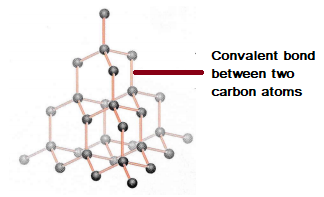
42 diagram of a carbon atom
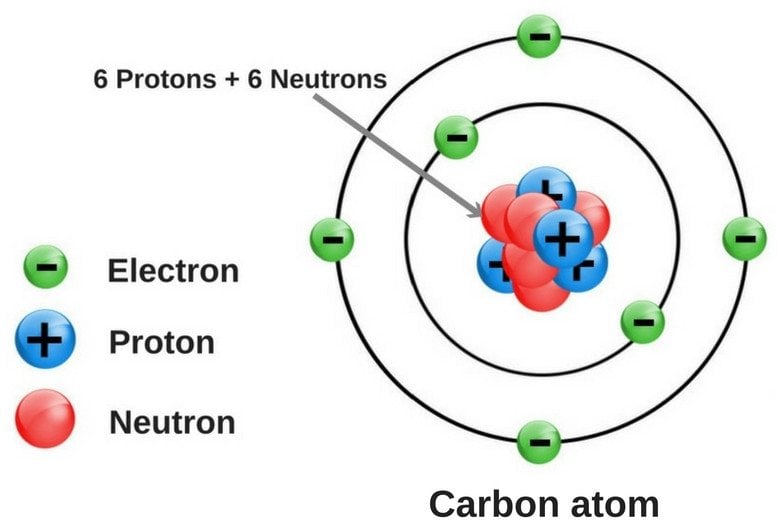
Diagram of a carbon atom? - Answers A diagram of a typical carbon atom would show a nucleus comprised of 6 protons and 6 neutrons. It would also have two electrons in the first energy level, then 4... The Lewis symbol for carbon: Each of the four valence electrons is represented as a dot. Which of the following is the correct Lewis dot structure for carbon dioxide CO2? So let’s form a double bond between the other oxygen and the carbon. Now, each atom in our structure has a full octet. So this is the correct Lewis structure for CO2.
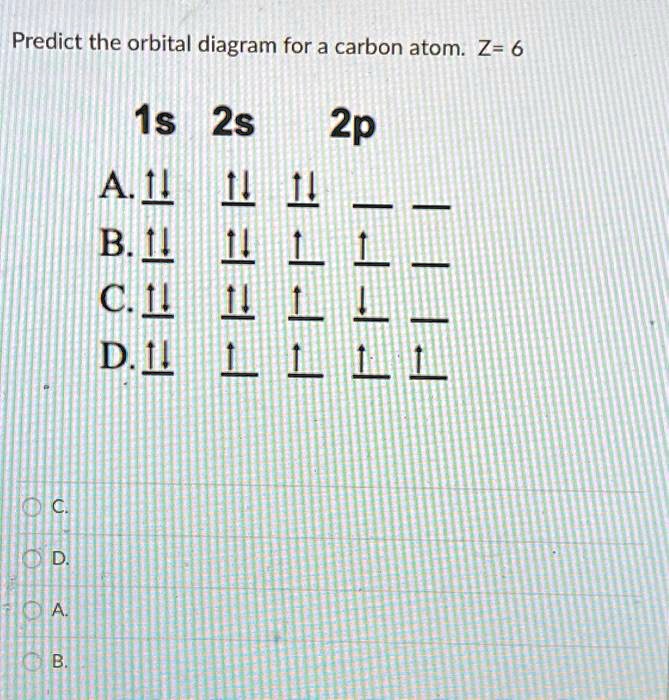
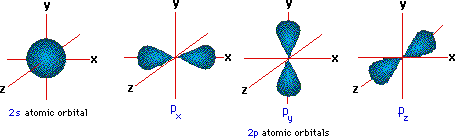
Carbon has a full 1s orbital, a full 2s orbital, and 2 electrons in the 2p orbital. It's electron configuration looks like this: 1s2 2s2 2p2 What is a molecular orbital diagram? A molecular orbital...
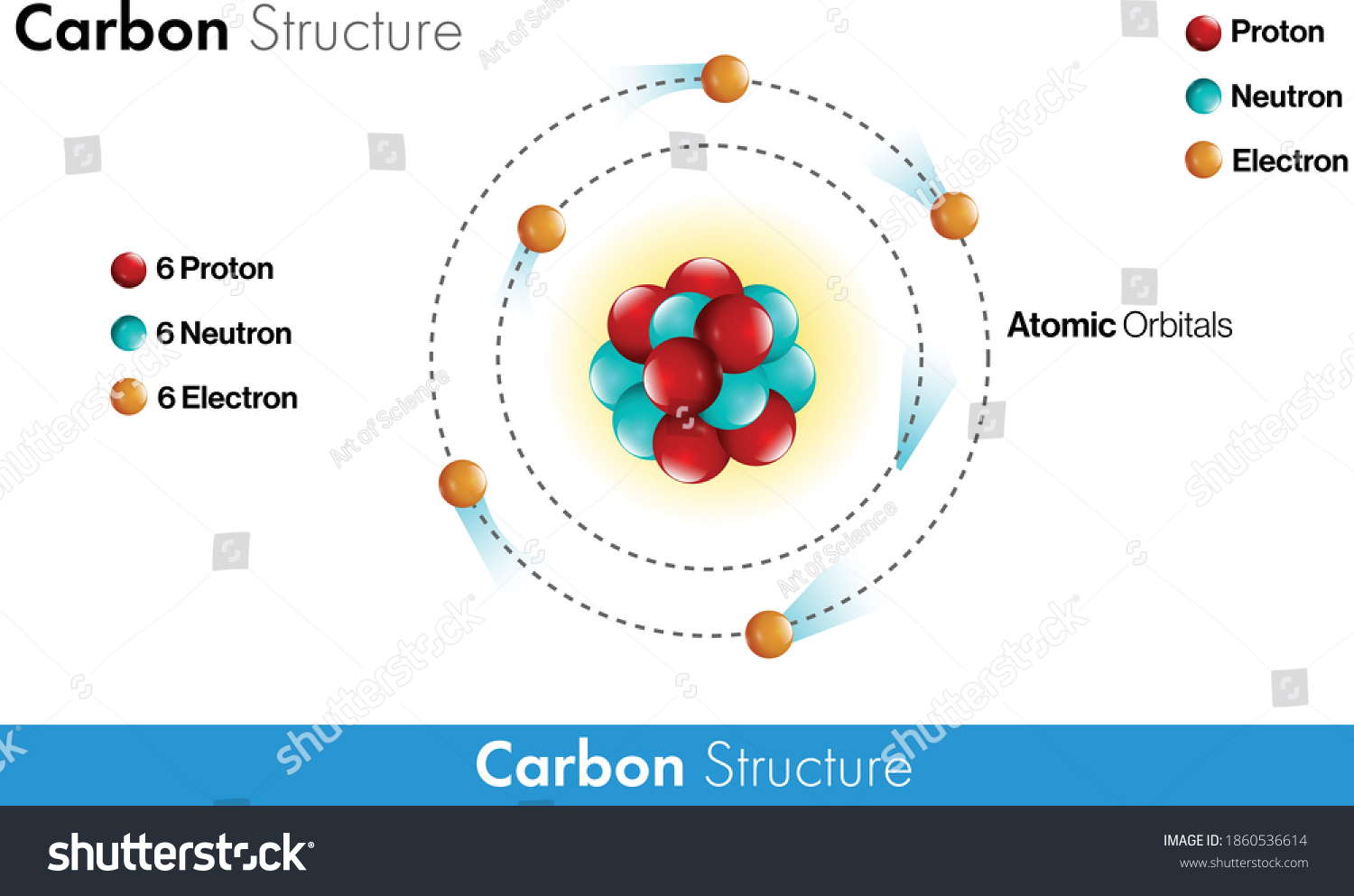
Diagram of a carbon atom
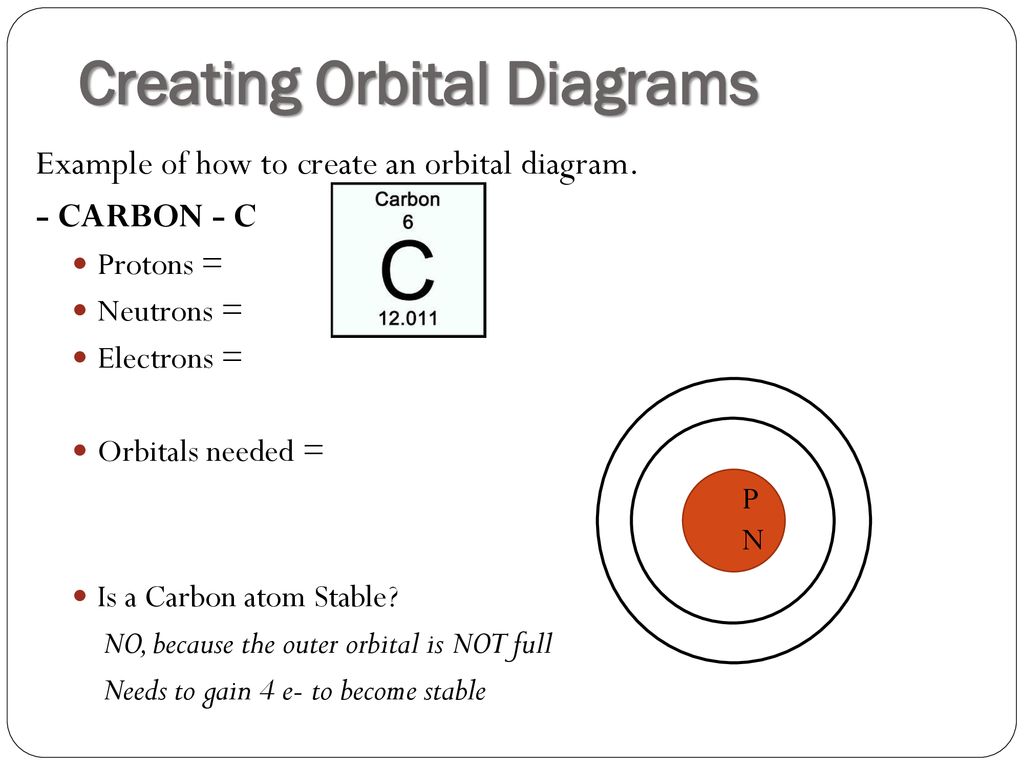
Draw and explain the molecular orbital diagram of carbon molecule. chemical bonding; class-11; Share It On Facebook Twitter Email 1 Answer +1 ... selected Dec 24, 2020 by Aashi01 . Best answer. 1. Electronic configuration of C atom – 1s 2 2s 2 2p 2. 2. Electronic configuration of C molecule is σ1s 2 σ*1s 2 σ2s 2 σ*2s 2 π2p x 2 π2p y 2 ... What is the orbital diagram for carbon atom in a ground state? By Hund’s rule, the electron configuration of carbon, which is 1s2 2s2 2p2, is understood to correspond to the orbital diagram shown in c. Experimentally, it is found that the ground state of a neutral carbon atom does indeed contain two unpaired electrons. The exercises present in Chapter 4 of NCERT Solutions for Class 9 Science are –. Number 4.1 – Charged particles in matter 2 Questions ( 2 short) Number 4.2 – The structure of an atom 4 Questions ( 4 short) Number 4.2.4 – Neutrons 2 Questions ( 2 short) Number 4.3 – How are electrons distributed.
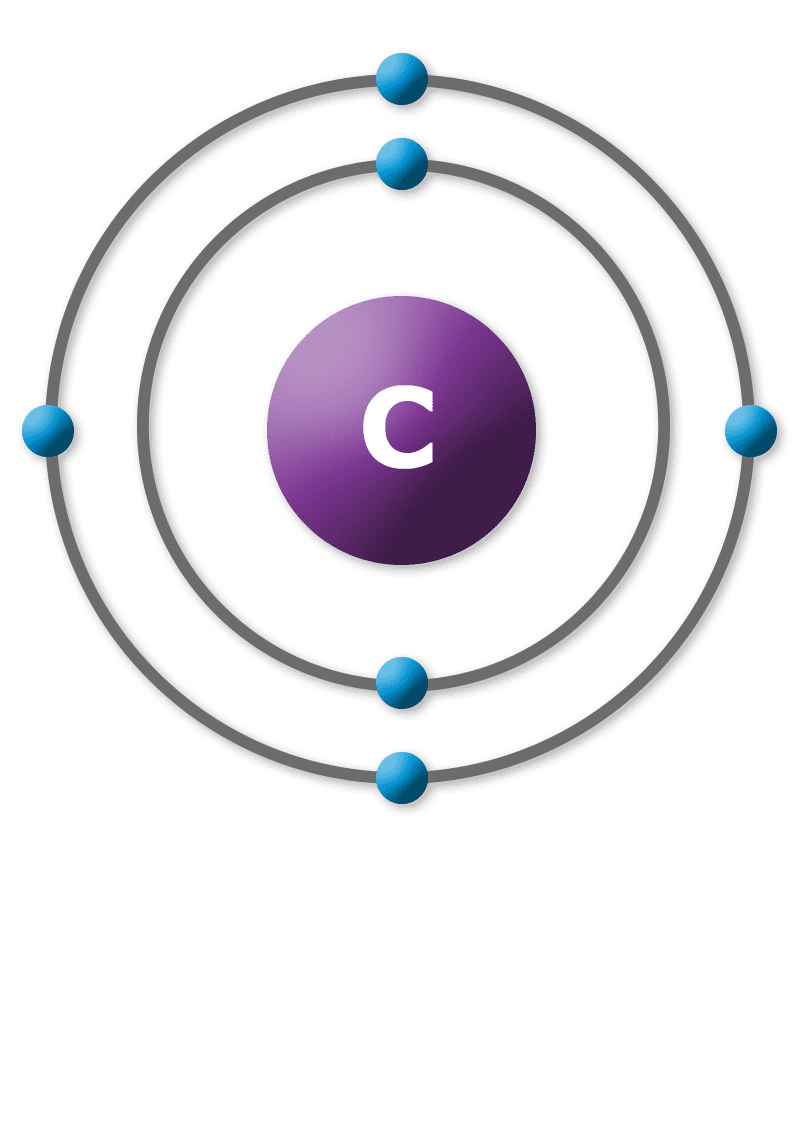
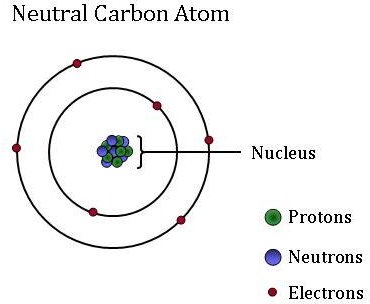
Diagram of a carbon atom. Download 143 Carbon Atom Diagram Stock Illustrations, Vectors & Clipart for FREE or amazingly low rates! New users enjoy 60% OFF. 178,403,224 stock photos online. In this article, the structure of an atom, you have understood what an atom is, the parts of a bit, the properties of the fundamental particles of an atom, i.e., protons, electrons, and neutrons. With examples of carbon-nitrogen provided on this page, you can explain the structure of an atom. Thus, electron distribution of sodium ion will be 2, 8. The atomic number of an element is equal to the number of protons in its atom. Since, sodium atom and sodium ion contain the same number of protons, therefore, the atomic number of both is 11. Question 7. The given figure depicts the atomic structure of an atom of an element ‘X’. The complete energy level diagram of the carbon atom, including fine structure, is shown on the next page. (a) (4 points) For the ground state electron configuration, draw the microstate for electrons in the 2p orbitals with the lowest energy. Identify L, S, and J, showing your work. Fill in the appropriate term.
The exercises present in Chapter 4 of NCERT Solutions for Class 9 Science are –. Number 4.1 – Charged particles in matter 2 Questions ( 2 short) Number 4.2 – The structure of an atom 4 Questions ( 4 short) Number 4.2.4 – Neutrons 2 Questions ( 2 short) Number 4.3 – How are electrons distributed. What is the orbital diagram for carbon atom in a ground state? By Hund’s rule, the electron configuration of carbon, which is 1s2 2s2 2p2, is understood to correspond to the orbital diagram shown in c. Experimentally, it is found that the ground state of a neutral carbon atom does indeed contain two unpaired electrons. Draw and explain the molecular orbital diagram of carbon molecule. chemical bonding; class-11; Share It On Facebook Twitter Email 1 Answer +1 ... selected Dec 24, 2020 by Aashi01 . Best answer. 1. Electronic configuration of C atom – 1s 2 2s 2 2p 2. 2. Electronic configuration of C molecule is σ1s 2 σ*1s 2 σ2s 2 σ*2s 2 π2p x 2 π2p y 2 ...























Comments
Post a Comment