39 butane energy diagram
April 26, 2021 - stereoelectronics.org contains interactive molecular structures illustrating organic reaction mechanisms, how drug molecules bind to their targets and the structural analysis of organic stereoisomers The minimum energy conformer for both ethane and butane, the anti-staggered conformer with a dihedral bond angle of 180 degrees, was obtained by manually adjusting the molecule and then using the 'Minimize Energy' feature. The following energies were recorded and graphed using Excel: 1) TORSIONAL ...
Next: · Thermodynamics, PV Diagrams, Internal Energy, Heat, Work, Isothermal, Adiabatic, Isobaric, Physics · Conformation of Butane /Malayalam/ ...
Butane energy diagram
The Henry's Law constant for n-butane is estimated as 0.95 atm-cu m/mole (SRC) based upon its vapor pressure, 1820 mm Hg (1), and water solubility, 61.2 mg/l (2). This Henry's Law constant indicates that n-butane is expected to volatilize rapidly from water surfaces (3). ) in butane, the two methyl groups are in close proximity with the molecule, therefore the potential energy is at its highest. ... ) in butane, the methyl groups are farther apart and therefore the potential energy drops by 4.1 kcal/mol. n-Butane pyrolysis at temperatures between 700°C and 840°C generates mainly propylene, ethylene, methane, and hydrogen, with small quantities of ethane and traces of butenes (isomers) and carbon [62,63]. The main reactions taking place during pyrolysis of n -butane are the following: (2.1.45) C 4 H 10 → C 3 H 6 + CH 4 Δ H = + 16.9 kcal / mol
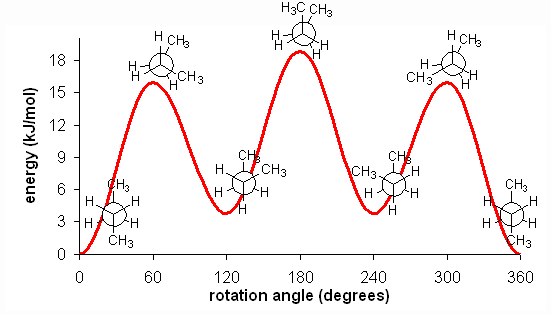
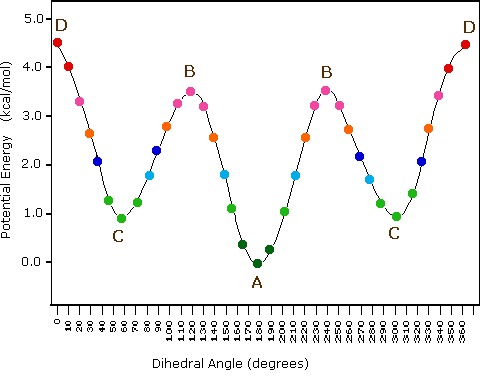
Butane energy diagram. Science. Chemistry. Chemistry questions and answers. What is the position of Gauche conformation in the energy diagram of butane? kcal/mol ENERGY (KI/mol) 6 25 14 3.3 w 3.2 o 0.8 0 -180 180 -120 -60 0 60 120 DIHEDRAL ANGLE ( BETWEEN CH3 GROUPS D ОА O B. Conformation of n-butane:- Butane may be treated as a derivative of ethane where one hydrogen on each carbon is replaced by a methyl group. The conformat ion of butane will be symmetrical only if the rotation will be about C 2-C 3 bond.. Butane has three conformations which are staggered (B, D, F). the symmetry of the molecule (e.g. in butane, H3C-CH2-CH2-CH3). A conformational energy diagram plotting the torsional angle against the energy results in the following profile: Two different eclipsed conformations and two staggered ones can be distinguished. The conformations are called rotamers, as they can be interconverted by rotation. The Online calculators, figures and tables showing specific heat, Cp and Cv, of gasous and liquid butane, C4H10, at varying temperarure and pressure, SI and Imperial units.
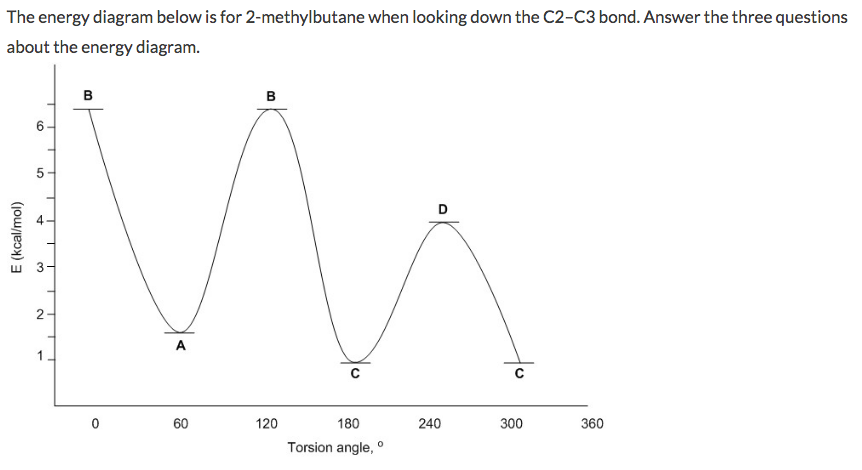
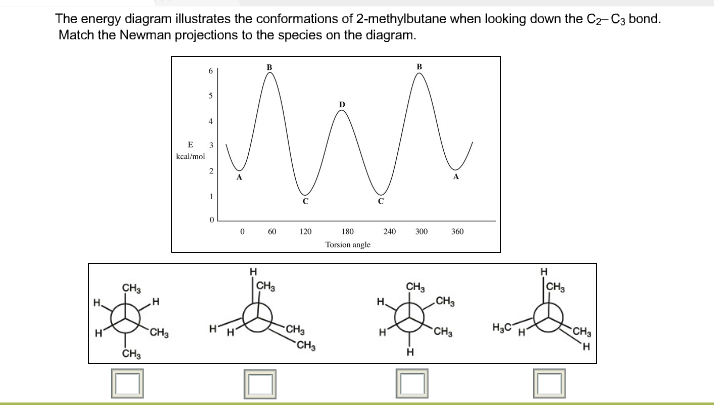
Does its energy diagram resemble the one for ethane or butane? Q. A. Sketch the curve showing the energy changes that arise from rotation about the C2-C3 bond of 2,2-dimethylpropane. B. Label the axes of your graph.... Q. Draw the lowest and the highest energy Newman projections for 2,2- dimethylbutane looking down the C2-C3 bond as shown. I've been unable to find an energy diagram for propane either in my book or online via Google. So I resorted to making my own. I suspect that propane isn't covered because it's essentially the same deal as ethane - we can have one staggered conformation and one eclipsed conformation (only in propane it's a methyl eclipsing a hydrogen, not hydrogens eclipsing each other). molecular orbital diagram as a non-bonding molecular orbital. 7. There are a total of 6 electrons to add to the molecular orbital diagram, 3 from boron and 1 from each hydrogen atom. sp Hybrid Orbitals in BeH2 1. The Lewis structure shows that the beryllium in BeH 2 makes 2 bonds and has no lone pairs. It is a linear molecule. Chemistry 104 ... Download scientific diagram | Total energy of the explosion for propane and butane vessels without PSV or a leak, subjected to fire, in the case of BLEVE: (]) full engulfment, W ) 8 mm, propane ...
Butane (/ ˈ b juː t eɪ n /) or n-butane is an alkane with the formula C 4 H 10.Butane is a gas at room temperature and atmospheric pressure. Butane is a highly flammable, colorless, easily liquefied gas that quickly vaporizes at room temperature. The name butane comes from the roots but-(from butyric acid, named after the Greek word for butter) and -ane.It was discovered by the chemist ... Butane is an alkane with the presence of C-C bonds. Normally, when we rotate the molecule of butane at the axis of the C-C bond, it shows different conformation isomerism. Generally, Butane has four conformation isomers which are fully eclipsed, gauche, eclipsed, and anti butane conformational isomers. 22. okt. 2021 ... All about the six key conformations of butane (drawn as Newman ... We can even graph the energy of the different conformations in 60 degree ... Butane is an alkane with the chemical formula , as shown in Figure 2.As a type of hydrocarbon, it can undergo hydrocarbon combustion which releases heat energy. Butane is one of the hydrocarbon components of raw natural gas, which is a type of fossil fuel. Butane is usually removed from natural gas before being shipped to customers, but then butane is sold separately as a fuel itself.
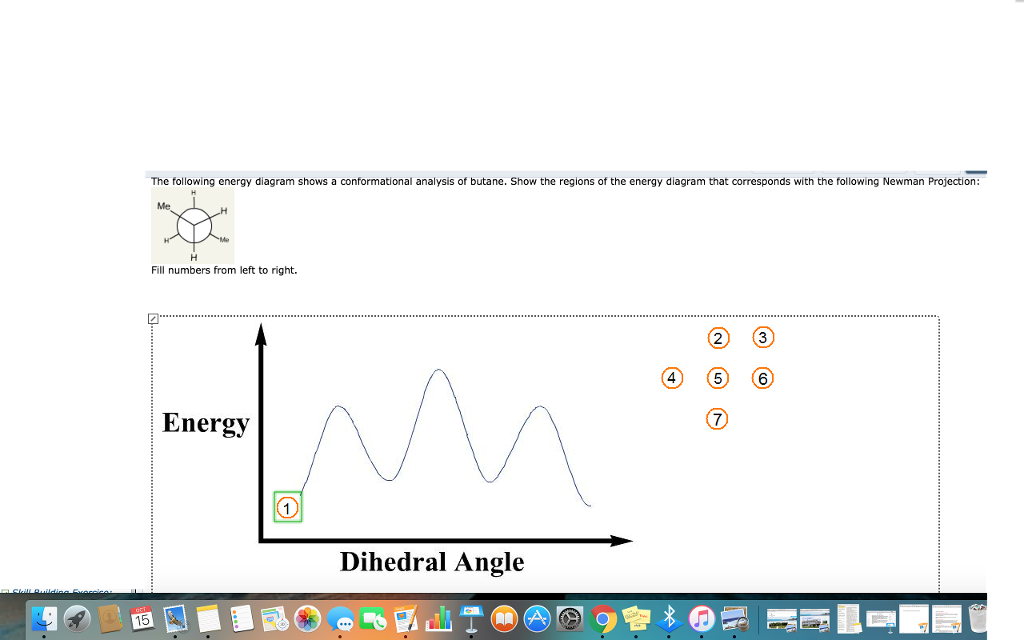
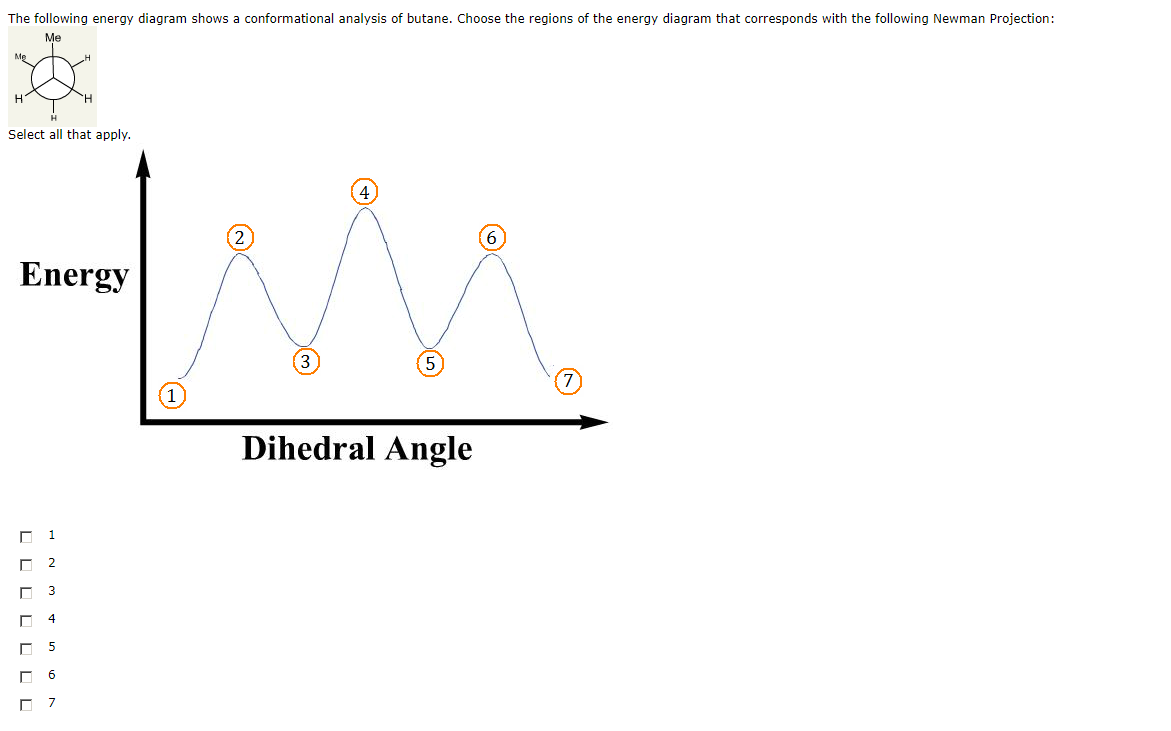
The following energy diagram shows a conformational analysis of butane. Show the regions of the energy diagram that corresponds with the following Newman Projection: Me 攻 Fill numbers from left to right. Energy 7 Dihedral Angle. Question: The following energy diagram shows a conformational analysis of butane.
, The heat capacity and entropy, heats of fusion and vaporization and the vapor pressure of n-butane, J. Am. Chem. Soc., 1940, 62, 1917-1923. [ all data ] Majer and Svoboda, 1985
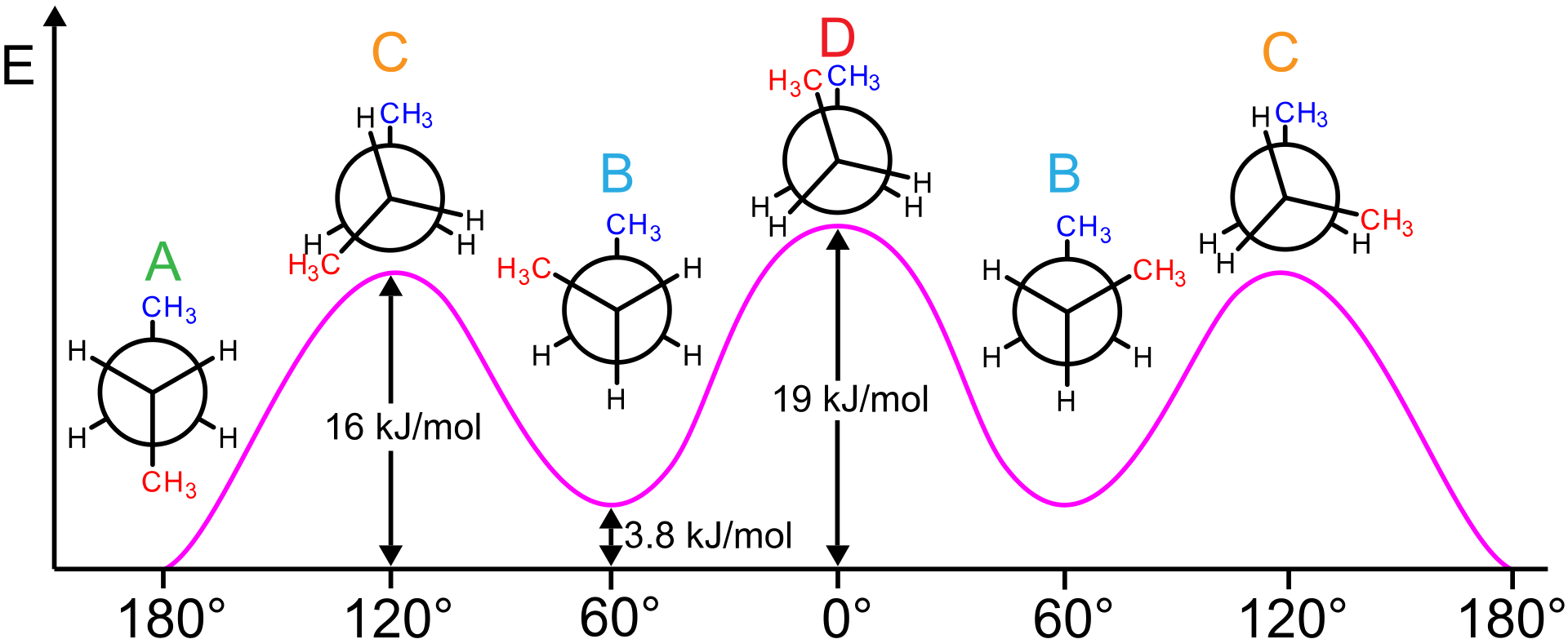
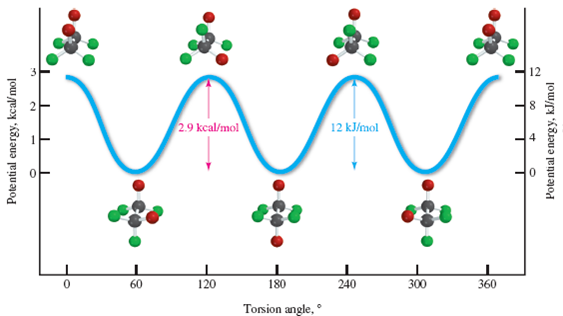
A CH3-CH3 eclipsing interaction is 17 KJ/mol. CH3 - H CH3 - CH3 * Energy diagram for the rotation of butane Summary: H - H eclipsed 4.0 KJ/mol torsional strain H - CH3 eclipsed 5.0 KJ/mol mostly torsional strain CH3 - CH3 eclipsed 17 KJ/mol torsional + steric strain CH3 - CH3 gauche 3.0 KJ/mol steric strain * 3.3: Conformations of Higher ...
butane. A very useful drawing convention is the Newman projection, named after the chemist who invented it in the 1960s. To draw a Newman projection, use the following process: Draw a circle. bond down which we look. If the front carbon is sp3-hybridized, draw three lines that meet at the center of the circle, 120° apart. (An sp2carbon
Butane Conformational Analysis. Butane Conformational Energy Diagram. There are two energy minima, the gauche and anti forms, which are both staggered and thus have no torsional strain. The anti form is the absolute energy minimum, since the gauche form has a small steric interaction between the two methyl groups.
We mentioned previously that hydrogen gas was the most efficient of all fuels in terms of heat emitted per gram of fuel burned. Gasoline is less efficient by nearly a factor of three, as the table at the right shows. Hydrogen gas releases 34 kcal per gram upon combustion in air; gasoline yields ...
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ...
March 23, 2017 - Firstly, you need to understand what is happening. Butane gas is burnt which releases heat energy. This heat energy is absorbed by water. As the water absorbs this heat energy, it increases in temperature from $\mathrm{7.44}$ to $\mathrm{50.7}$ degrees Celsius.
R600 (Butane) Pressure enthalpy chart. Scroll down to find download buttons for pressure enthalpy charts in either Metric or Imperial Units. Downloads as a PDF.
In physics, energy density is the amount of energy stored in a given system or region of space per unit volume. It may also be used for energy per unit mass, though a more accurate term for this is specific energy (or gravimetric energy density). Often only the useful or extractable energy ...
CID 18696402 | C15H36 | CID 18696402 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety ...
Conformations of butane. The conformational possibilities increase as alkanes become larger. A plot of potential energy against rotation about the C(2)-C(3) bond in butane is shown below. The lowest-energy arrangement, called the antiperiplanar (or anti) conformation, is the one in which the two large methyl groups are as far apart as possible.As rotation around the C(2)-C(3) bond occurs ...
Rotation of anti butane by 120 degrees again leads to the formation of the gauche conformer. ... The ranking of the conformation isomers is given below taking into account the energy levels from lowest to highest. It is given as; ... This can also be visualized using an energy diagram.
In order to discover the change in energy, steric strain summary was computed from the MM2 menu and recorded. For ethane, torsion, 1,4-VDW and total energy were recorded at each 15 o rotation of the dihedral angle. For butane, torsion, non-1,4-VDW, 1,4-VDW and total energy were recorded for every 15 o rotation.

some lights in one of my favorite coffeehouse that i disciverd thanks to my sister. it captures that feeling that i had on bali back in may, how i miss this palce
, The entropies of n-butane and isobutane, with some heat capacity data for isobutane, J. Chem. Phys., 1937, 5, 359-363. ... Appearance energy: C p,gas: Constant pressure heat capacity of gas: C p,liquid: Constant pressure heat capacity of liquid: IE (evaluated) Recommended ionization energy: P c:
Energy densities (LHV) for fuels in liquid state [pdf]. Sandia National Laboratories. George Thomas, BES workshop May 13, 2003: 9. ... Butane is a colorless, flammable hydrocarbon with an empirical formula C4H10. It has the odor of a natural gas, is extremely stable, has no corrosive action ...
September 25, 2017 - Answer (1 of 2): It has to do with density or specific gravity. I’ve seen any number of articles saying that butane has more energy content and is, therefore, more economical to use. Not always true! It actually depends on the unit of measure used for pricing. If it is priced by volume — in l...
The below figure represents the 4 conformations of butane. The above diagram explains the rotation about C2-C3 bond due to the change in potential energy. Uses of Butane: Pure of butane can be used as a refrigerant. It is used in Butane Torch. It is widely used in gasoline blending. Butane Cartridges are used to powered cordless hair irons.
Conformational Analysis of n-Butane In this exercise you will calculate the Molecular Mechanics (MM) single point energy of butane in various conformations with respect to internal rotation around the C 2-C 3 bond and generate Excel charts of steric energy vs. dihedral angle. You will decide which factor contributes most to
Eclipsed conformation IV has the greatest energy of all because of the added large repulsive dispersion forces between the eclipsed methyl groups as compared to II and VI. ** Although the barriers to rotation in a butane molecule are larger than those of an ethane molecule, they are still far too small to permit isolation of the gauche and anti ...

All wind turbines side by side producing pure electricity without destroying our beloved planet Earth.
Butane is a gas at standard conditions. However, at lower temperature and/or high pressures the gas becomes a liquid or a solid. The butane phase diagram shows the phase behavior with changes in temperature and pressure. The curve between the critical point and the triple point shows the butane boiling point with changes in pressure.
Here's an energy diagram showing the different conformations we saw in the video. And these pictures are just stills from the actual video. We started with the staggered conformation of butane right here, which has a certain potential energy, and we went from this staggered conformation to this eclipsed conformation right here by rotating 60 ...
This is an energy diagram of the conformations of n-Butane calculated using Spartan from Wavefunction. The 2,3 bond was set for torsional angles starting at ...
You should be able to draw these conformations and this diagram and attach the appropriate numerical energies to each conformation. You should also be able to identify the type or types of strain present in each conformation and explain the basis of that strain.
Comparing fuels using heat content, suitability, and products of combustion, tutorial with worked examples for chemistry students.
September 27, 2021 - As a type of hydrocarbon, it can undergo hydrocarbon combustion which releases heat energy. Butane is one of the hydrocarbon components of raw natural gas, which is a type of fossil fuel.[2] Butane is usually removed from natural gas before being shipped to customers, but then butane is sold ...
September 13, 2020 - Since the staggered conformers represent the chief components of a butane sample they have been given the identifying prefix designations anti for A and gauche for C. ... The following diagram illustrates the change in potential energy that occurs with rotation about the C2–C3 bond.
Since the specific energy, 2 2 2 2 2gA Q y g V E =y + = + Figure 5.1. Specific energy diagram For a channel of known geometry, E = f (y, Q). Keeping Q = constant = Q1, the variation of E with y is represented by a cubic parabola. (Figure 5.1). It is seen that there are two
8. feb. 2021 ... ... correlate energies of conformations with rotational energy diagrams and predict the most stable conformations for butane.
Professor Davis demonstrates the conformational energy diagram of butane using Newman projections. Anti, syn, guache and staggered conformations are all dem...
The energy diagram for carbon in CO 2 is shown below. What is the hybridization of oxygen in CO 2. Each oxygen has two lone pairs and forms one s bond and one p bond. This means that there must be three hybridized orbitals and one unhybridized p orbital to make the p bond. This is sp 2 hybridization.
n-Butane pyrolysis at temperatures between 700°C and 840°C generates mainly propylene, ethylene, methane, and hydrogen, with small quantities of ethane and traces of butenes (isomers) and carbon [62,63]. The main reactions taking place during pyrolysis of n -butane are the following: (2.1.45) C 4 H 10 → C 3 H 6 + CH 4 Δ H = + 16.9 kcal / mol
) in butane, the two methyl groups are in close proximity with the molecule, therefore the potential energy is at its highest. ... ) in butane, the methyl groups are farther apart and therefore the potential energy drops by 4.1 kcal/mol.
The Henry's Law constant for n-butane is estimated as 0.95 atm-cu m/mole (SRC) based upon its vapor pressure, 1820 mm Hg (1), and water solubility, 61.2 mg/l (2). This Henry's Law constant indicates that n-butane is expected to volatilize rapidly from water surfaces (3).















Comments
Post a Comment