40 neon dot diagram
1 answerThe Lewis dot structure of Neon is shown below. Neon is a noble gas that belongs to Group 8A of the periodic table. This means that it has a... Draw a Lewis Dot Diagram for oxygen. OGT Questions _____1. The noble gas neon is used for filling neon signs. Like other noble elements, it has a full octet (complete outer energy level) of electrons, which makes the gas A. freeze at room temperature. B. react ...
Each oxygen atom will share 2 of its valence electrons in order to form 2 bonding pairs of electrons (a double covalent bond) so that each oxygen atom will have a share in 8 valence electrons (electronic configuration of neon). Lewis Structure (electron dot diagram) for the oxygen molecule, O 2, OR

Neon dot diagram
Founded in 2002 by Nobel Laureate Carl Wieman, the PhET Interactive Simulations project at the University of Colorado Boulder creates free interactive math and science simulations. PhET sims are based on extensive education <a {0}>research</a> and engage students through an intuitive, game-like environment where students learn through exploration and discovery. Solution. Having lost its two original valence electrons, the Lewis electron dot diagram is just Ca 2+. Ca2+. The O 2− ion has gained two electrons in its valence shell, so its Lewis electron dot diagram is as follows: Test Yourself. The valence electron configuration of thallium, whose symbol is Tl, is 6 s2 5 d10 6 p1. Neon is a chemical element with a chemical symbol Ne and atomic number 10. It is a noble gas that is colorless, odorless, inert and monatomic. It is the fifth most abundant chemical element in the universe by mass but a rare element on Earth. It displays a reddish-orange light, and is commonly used in low-voltage neon glow lamps, high-voltage ...
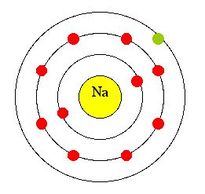
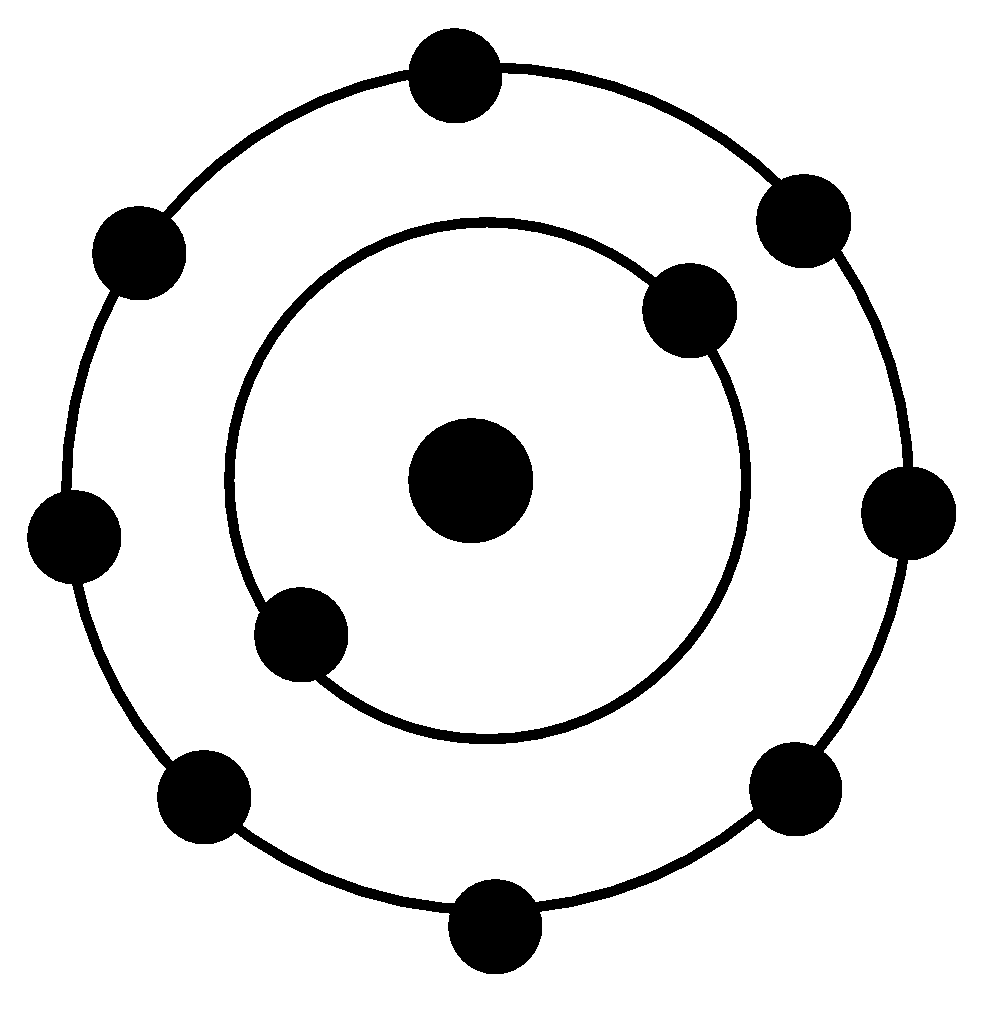
Neon dot diagram. Neon (Ne). Diagram showing the nuclear composition and electron configuration of an atom of neon-20 (atomic number: 10), the most common isotope of the element neon. The nucleus consists of 10 protons (red) and 10 neutrons (blue). Ten electrons (green) bind to the nucleus in shells (rings), filling the outer (second) electron shell in what is a very stable configuration. The stability of an ... 25.4.2021 · Look at the last image at the top of the page. The circles are right next to each other so that the dot at the end of one circle is actually closer to the dot at the end of the neighboring circle. But despite how close those two dots are, we see the dots inside the circles as … 12 Jul 2021 — Neon Lewis Dot Structure What does an electronic dot diagram tell you? Well, Lewis point structures, or electron dot diagrams, ... Neon Part 2 answers: Total El e s No. of Valence El e s Chemical Symbol Total Electrons No. of Valence Electrons D Page 27 of 44 Dot Diagram Chemical for Compound Formula Elements Dot Diagram for Each Element Na and F Br and Br Mg and O Formed Nat Mg:ë: NaF . Author: Bill Puckett
Neon has 2 electrons in its first shell and 8 in its secondCheck me out: http://www.chemistnate.com Examples for Drawing Lewis Dot Structure for Covalent Bonds . Here, we will be using the determined total number of valence electrons per atom and drawing them in the proper places. Reference the "How to Draw a Lewis Dot Structure" for a Step by Step guide. See the following Lewis dot structure diagrams for a few covalent compounds. Example 1. Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation.It is a form of luminescence.In most cases, the emitted light has a longer wavelength, and therefore a lower photon energy, than the absorbed radiation. A perceptible example of fluorescence occurs when the absorbed radiation is in the ultraviolet … A. Lewis electron dot diagram. (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the ...
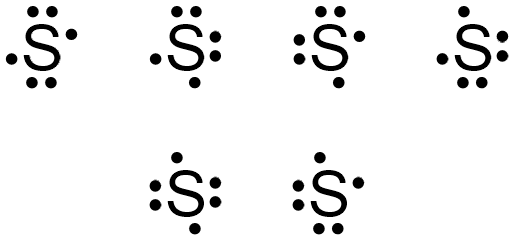
3LCD is the name and brand of a major LCD projection color image generation technology used in modern digital projectors. 3LCD technology was developed and refined by Japanese imaging company Epson in the 1980s and was first licensed for use in projectors in 1988. In January 1989, Epson launched its first 3LCD projector, the VPJ-700. Although Epson still owns 3LCD … A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side. Atomic Structure of Neon. Atomic Radius: 0.51 Å. Atomic Volume: 16.7cm 3 / mol. Covalent Radius: 0.71 Å. Cross Section (Thermal Neutron Capture) σ a / barns: 0.04. Crystal Structure: Cubic face centered. Electron Configuration: 1s 2 2s 2 p 6. Electrons per Energy Level: 2,8. Lewis dot structure of noble gases. First we will see how to draw Lewis dot structures for noble gases. Let's start with helium. Its atomic number is 2 and electronic configuration is 1s 2. It contains 2 electrons in K shell. Next, neon. It has atomic number ten and two shells such as k and L. Each shell is loaded with maximum number of ...
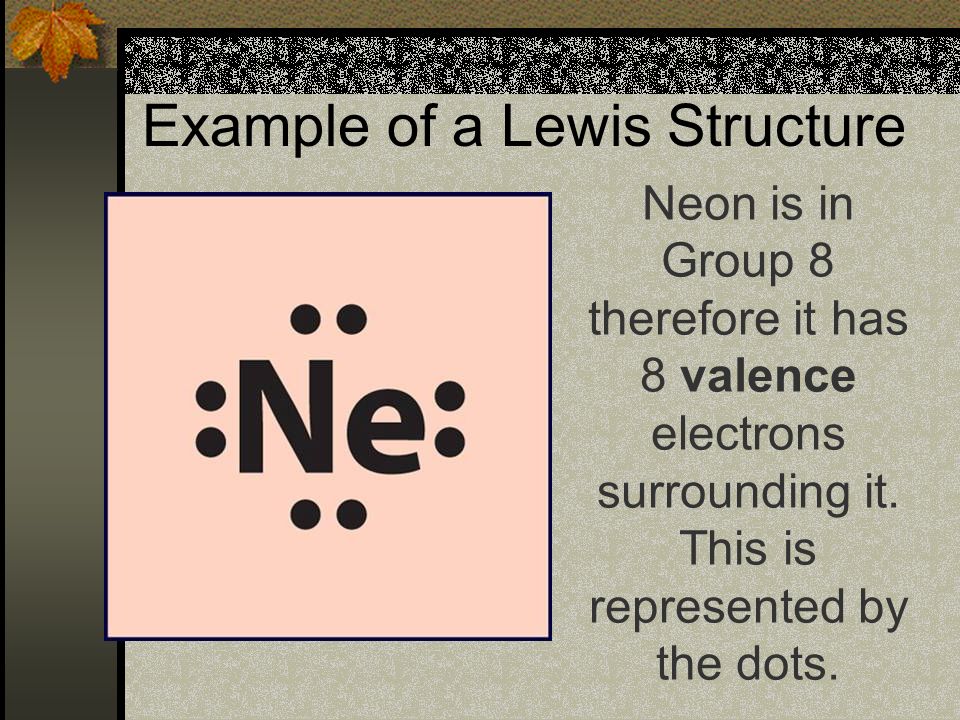
Electron dot diagram of a Neon atom. Electron dot diagram also called lewis structure which represents the valence electrons of atoms. As, from the Bohr diagram of Neon, we got to know, it has 8 valence electrons. So, just represent these 8 valence electrons around the Neon atom as a dot. The electron configuration of Neon “Electron configuration is the distribution of electrons of an atom ...
A Lewis electron dot diagram (or electron dot diagram, or a Lewis diagram, or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side. (The ...
An atom of Neon can be represented by the diagram on the left. But in this case, we are drawing electronic dot diagrams. For neon, we must determine a) number of valence electrions b) place dots around the element to represent the valence electrons.
Find & Download Free Graphic Resources for Circuit. 37,000+ Vectors, Stock Photos & PSD files. Free for commercial use High Quality Images
The electron dot diagram of helium has six fewer dots than the electron dot diagram of neon. The electron dot diagram of helium has eight fewer dots than the electron dot diagram of neon. Both the electron dot diagram of helium and the electron dot diagram of neon have two dots.
Describe the electron dot diagram system of representing structure. Draw electron dot diagrams for elements. Electron Dot Diagrams. lithium 1 s 2 2 s 1 1 valence electron nitrogen 1 s 2 2 s 2 2 p 3 5 valence electrons neon 1 s 2 2 s 2 2 p 6 8 valence electrons
Lewis Dot Structures Objectives: 1. Draw Lewis structures for atoms, ions and simple molecules. ... An energy level diagram is shown below. ... This way all three electrons can have the same spin. For oxygen, fluorine and neon, the fourth, fifth and sixth electrons, respectively, are added to the half-filled orbitals.
18.2.2019 · The Lewis Dot Structure for carbon dioxide can be represented like this: ... In the second part of drawing a Lewis dot diagram, ... These elements are referred to as the noble gases, and they include elements like argon, neon, and krypton.
2b) For an electron dot diagram, how many dots would be shown around the noble gas elements (except for helium)? 2c) Neon has 10 electrons in all and argon has 18, yet the electron dot diagrams for both neon and argon are the same. Explain why. 3. In the diagram below, some electrons are coloured red and some black. What name is given to
Mr. Andersen shows you how to draw Lewis Dot Diagrams for atoms and simple molecules.Intro Music AtributionTitle: I4dsong_loop_main.wavArtist: CosmicDLink to...
The dot diagram for Neon is made up of two shells with the first shell having two 'dots' and the second having eight. This because the atomic number is ten. How to draw it: Have one dot in the ...
Now most people will represent this dot structure by putting brackets here. And putting a positive charge outside of it. So there's your xenon pentafluoride cation. So we're going to a lot more examples for drawing dot structures in the next several videos, and see how drawing dot structures allows you to predict the shapes of different molecules.
23 Jan 2021 — The electronic configuration of neon is 1s2 2s2p6. The valence electrons dot diagram is a diagram where valence electrons of atoms are ...
Electron Dot Diagrams. a picture that represents the number of valence electrons in an element. Nice work! You just studied 5 terms! Now up your study game with Learn mode.
in the diagram above and the situation shown in the diagram below. Another molecule which exhibits resonance is SO 3. You should be able to fi ll in the missing diagrams. And then you write the Lewis/line resonance diagrams as: Now try to form the Lewis diagram for benzene, C 6 H 6, where the carbon atoms form a ring. Expect reso-nance.
The electron dot diagram which is also known as the Lewis electron dot diagram is a diagram that shows an atom's valence electrons by placing dots, which represent the valence electrons of the element, around the elements symbol. The element for which the electron dot diagram is found = Neon, Ne. The atomic number of neon, Ne = 10.
2 7 Applications Of Electron Configurations Valence Electrons And Electron Dot Structures Chemistry Libretexts
23.11.2021 · Now, we will proceed to draw a simple sketch or skeletal diagram of cyanide ion. We have placed both the carbon and nitrogen atoms as atomic symbols here. We will henceforth place the electron dot notations. We have put all the 10 valence electrons surrounding the constituent atoms of the CN ionic molecule. Here comes the concept of octet ...
Neon is the tenth element with a total of 10 electrons. In writing the electron configuration for neon the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Ne go in the 2s orbital. The remaining six electrons will go in the 2p orbital. Therefore the Ne electron configuration will be 1s 2 2s 2 2p 6. Because the second energy level (2s ...
There are two types of diagrams one is the Lewis diagram the other is the Electron dot diagram. To make the electron dot diagram you put the electron symbol and put a dot on o ne of the sides for each period (you don't count the middle section.)Ne: = Neon dot diagram ' '.
The electron dot diagram of helium has six fewer electrons than the electron dot diagram of neon. Recommended textbook explanations. Interactive Science: Physical Science. 1,559 explanations. Modern Chemistry 1st Edition Sarquis, J., Sarquis, M. 2,183 explanations.
What is the Lewis dot structure for neon? Electron Dot Diagrams. lithium 1 s 2 2 s 1 1 valence electron; beryllium: 1 s 2 2 s 2: 2 valence electrons: nitrogen: 1 s 2 2 s 2 2 p 3: 5 valence electrons: neon: 1 s 2 2 s 2 2 p 6: 8 valence electrons: What do the dots around the element symbol represent? Lewis dot diagrams use dots arranged around the atomic symbol to represent the electrons in the ...
Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element's symbol. A beryllium atom, with two valence electrons, has the electron dot diagram below: (8.1.1) ⋅ Be ⋅. Since electrons repel each other, the dots for a given atom are distributed evenly around the symbol before ...
A. The electron dot diagram of helium has six fewer electrons than the electron dot diagram of neon. B. The electron dot diagram of helium has eight fewer electrons than the electron dot diagram of neon. C. Both the electron dot diagram of helium and the electron dot diagram of neon have two dots. D. Both the electron dot diagram of helium and ...
This is the correct dot diagram for sodium, group 1. Q. This is the correct dot diagram for nitrogen, group 15. Q. This is a correct dot diagram for neon, group 18. Q. This could be the dot diagram of. Mg, group 2. Cl, group 17.
Neon is a chemical element with a chemical symbol Ne and atomic number 10. It is a noble gas that is colorless, odorless, inert and monatomic. It is the fifth most abundant chemical element in the universe by mass but a rare element on Earth. It displays a reddish-orange light, and is commonly used in low-voltage neon glow lamps, high-voltage ...
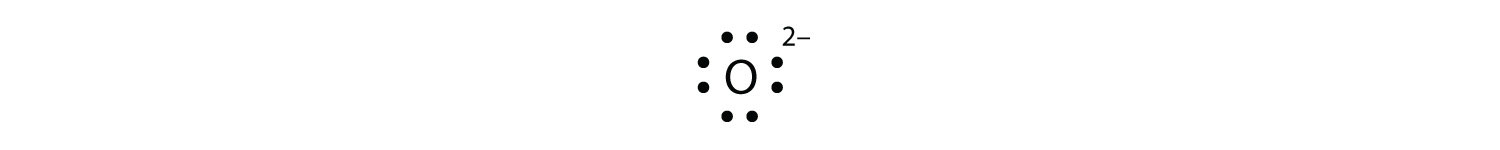
Solution. Having lost its two original valence electrons, the Lewis electron dot diagram is just Ca 2+. Ca2+. The O 2− ion has gained two electrons in its valence shell, so its Lewis electron dot diagram is as follows: Test Yourself. The valence electron configuration of thallium, whose symbol is Tl, is 6 s2 5 d10 6 p1.
Founded in 2002 by Nobel Laureate Carl Wieman, the PhET Interactive Simulations project at the University of Colorado Boulder creates free interactive math and science simulations. PhET sims are based on extensive education <a {0}>research</a> and engage students through an intuitive, game-like environment where students learn through exploration and discovery.




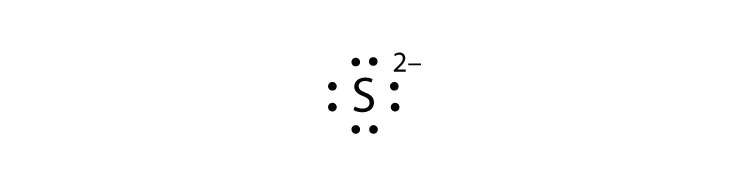












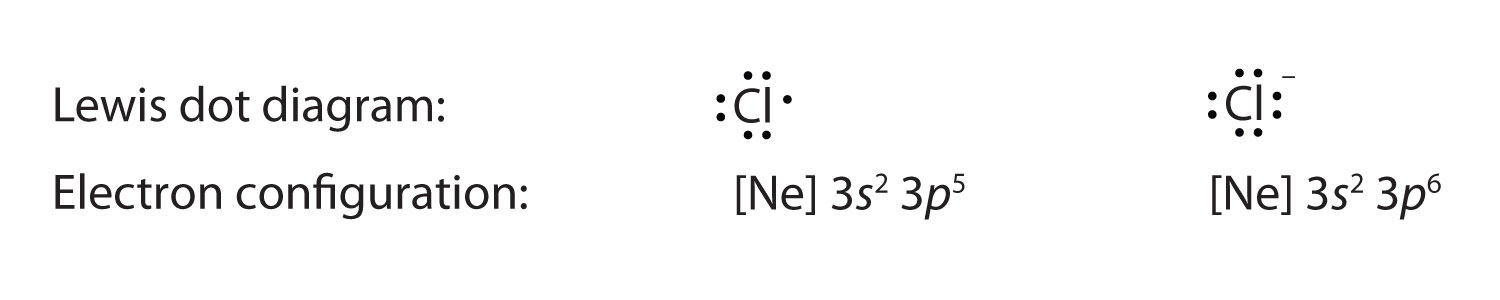








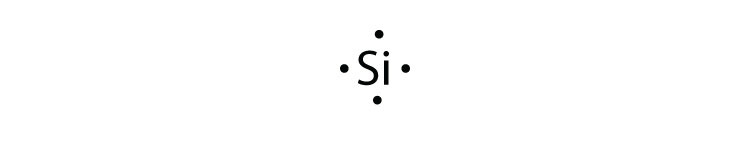

Comments
Post a Comment