39 orbital diagram worksheet with answers
Chemistry Unit 2 Worksheet 1: Electron Configurations 1. The further the electron is from the nucleus, the _____ energy the electron has. 2. A(n) _____ is often thought of as a region of space in which there is a high probability of finding an electron. 3. What is the term used to label the energy levels of electrons? _____ 4. the same period. Support your comparison with an orbital diagram. 1st IE of each halogen is lower than that of the noble gas element next to it in the same family. The orbital diagram will show one less e- for each halogen family and each noble gas family with full valence electron shell. A full shell is what the
Use the patterns within the periodic table to write the orbital notation for the following atoms. Symbol # e-Orbital Notation ... your answer for each. Hund's Rule, Pauli Exclusion Principle, ... Electron Configuration Worksheet

Orbital diagram worksheet with answers
Homework Worksheet #3: Electron Configurations ... Orbital Diagram Configuration Orbital Diagram Configuration . Dr. Mihelcic Honors Chemistry 2013-14 8 Homework Worksheet #7: Electron Configurations ... your answer for each. Hund's Rule, Pauli Exclusion Principle, Aufbau Principle ... -Orbital diagrams are visual representations of electron configuration . Hund's Rule •When electrons are filling orbitals of the same energy, they prefer to enter empty orbitals first. These electrons all have the same spin •A diagram of nitrogen is shown below (7 total The energy diagram for this process is shown below. The hybridized orbitals are higher in energy than the s orbital, but lower in energy than the p orbitals. atomic orbitals hybridized orbitals Carbon has 4 valence electrons. Add these electrons to the atomic and molecular orbitals. This hybridization gives tetrahedral geometry.
Orbital diagram worksheet with answers. other sigma-symmetric orbital, and • two sigma -symmetric AOs close enough in energy to combine: 1𝑠𝑠(𝐻𝐻) and 3𝑝𝑝. 𝑧𝑧 (𝐶𝐶). 𝑒𝑒. σ. 1 (c) (d) The Lewis diagram shows an 𝐻𝐻−𝐶𝐶 bond order of 1 (single bond)𝑒𝑒 . The 𝐻𝐻−𝐶𝐶 bond order𝑒𝑒 calculated from the MO diagram is . 2 ... We draw orbital diagrams to show the distribution of electrons in a sublevel. Circles are used to represent the individual _____. _____ are used to represent electrons in the orbital. The first electron in an orbital is represented by a ! and the second by a!. A set of four _____ numbers is assigned to each _____ to It's known as an orbital diagram or formally as an Aufbau diagram. Electron Configuration Worksheet pdf. Electron Configuration Worksheet Answer Key pdf. Exercise 1. Hydrogen (H) Hydrogen is element 1 on the periodic table with 1 electron when it's neutral. The idea is to draw an arrow for each electron, so in this case we just have one ... Electron Configuration Practice Worksheet Answers Promotiontablecovers : Write The Unabbreviated Electron Configurations Of The Following Elements Everett Community College Tutoring Center Student Support Services Program. Electron Sentences Worksheet , Use The Orbital Filling Diagrams To Complete The Table.
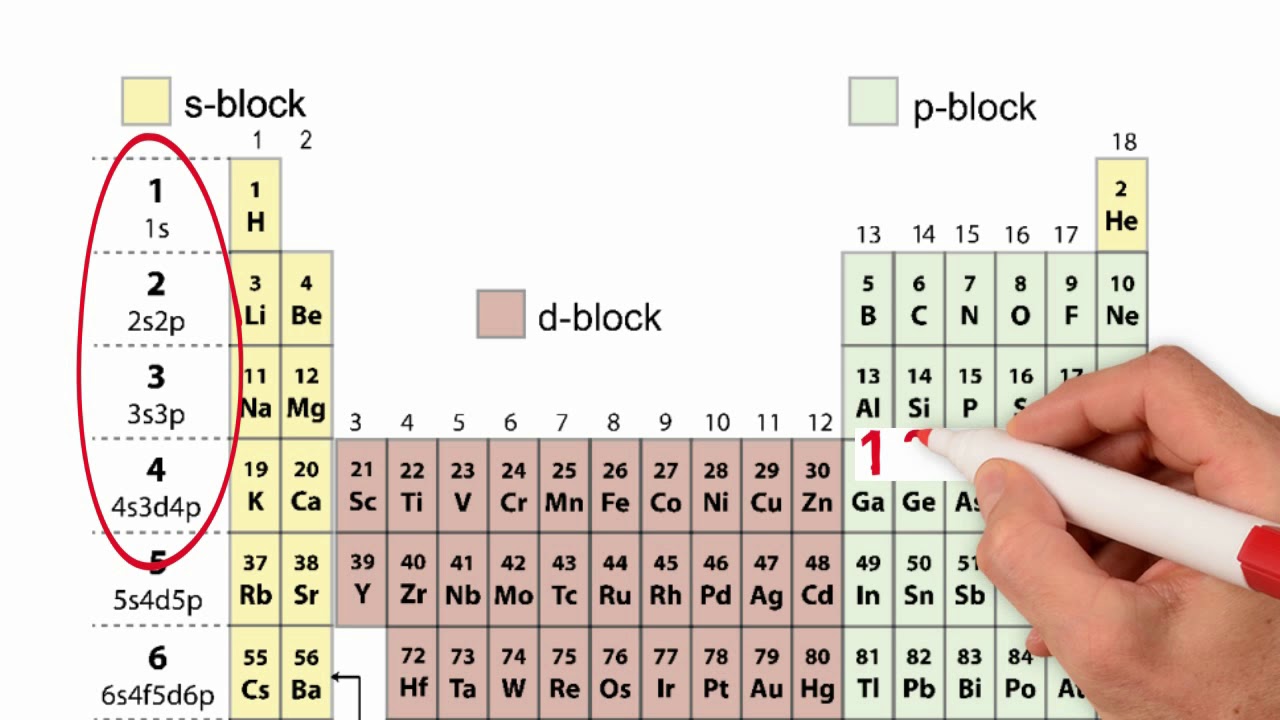
Orbital Filling Diagrams •Each box represents an orbital which can hold a max of 2 e- •Aufbau principal -each electron occupies the lowest energy orbital available; German for "build up" •Electrons are notated with an arrow -Up arrow goes first then, down arrow -Arrows represent the opposing spin of electrons 5.2 Quantum Theory & The Atom Central net force model worksheet 4: orbital motion 4. Ae worksheet orbital filling rules answers 15-16.pdf. Worksheets page 14 addicted to sugar worksheet answers orbital diagram chem worksheet 5 5 answers metric measurement worksheets 5th grade first grade activity worksheets division questions with remainders numbers worksheet for kg. Using the periodic table answer the following questions: 16) Which Groups have an s-orbital as the last orbital? 1 and 2. 17) Which Groups have a p-orbital as the last orbital? 13 - 18. 18) Which Groups have a d-orbital as the last orbital? 3 - 12. Which section of the table is left? What is trend of f-orbital elements? Lanthanide and actinide 8. The following diagram shows 5 different energy levels (n) that electrons can occupy. Use the diagram to answer each of the following questions: a. EXAMPLE Give a situation that could result in an electron making a quantum leap. An electron gains energy and moves from n=1 to n= 3. b. Give a situation that would result in an electron giving ...
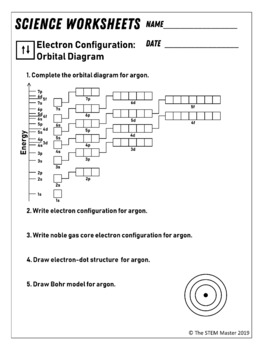
Electron configuration and orbital diagram worksheet. Electron configurations worksheet write the complete ground state electron configurations and orbital notations for the following. Phosphorus 1s 2s 2p 3s 3p 4s 3d 4p. Write orbital filling diagrams electron configurations and electron dot diagrams. O2 1s2 2s2 2p4. The 2px orbital lies on the x axis. This is one of many possible answers 5. Use the orbital filling diagrams to complete the table. This worksheet will help students understand how electrons fill into orbitals and how orbital diagrams are drawn using spdf configuration. Electron Orbitals: Electron Configuration Orbital Diagram Worksheet Answers (The electron configuration orbital diagram worksheet answers can be found at the bottom of the lesson.) The 2, 8, 8, 18 rule is a very simplistic view of electron configuration and doesn't give the full picture when it comes to electron configuration. Science Chemistry library Electronic structure of atoms Electron configurations. Electron configurations. Shells, subshells, and orbitals. Introduction to electron configurations. Noble gas configuration. Electron configurations for the first period. Electron configurations for the second period. Electron configurations for the third and fourth ...
Created Date: 2/18/2015 7:50:23 AM
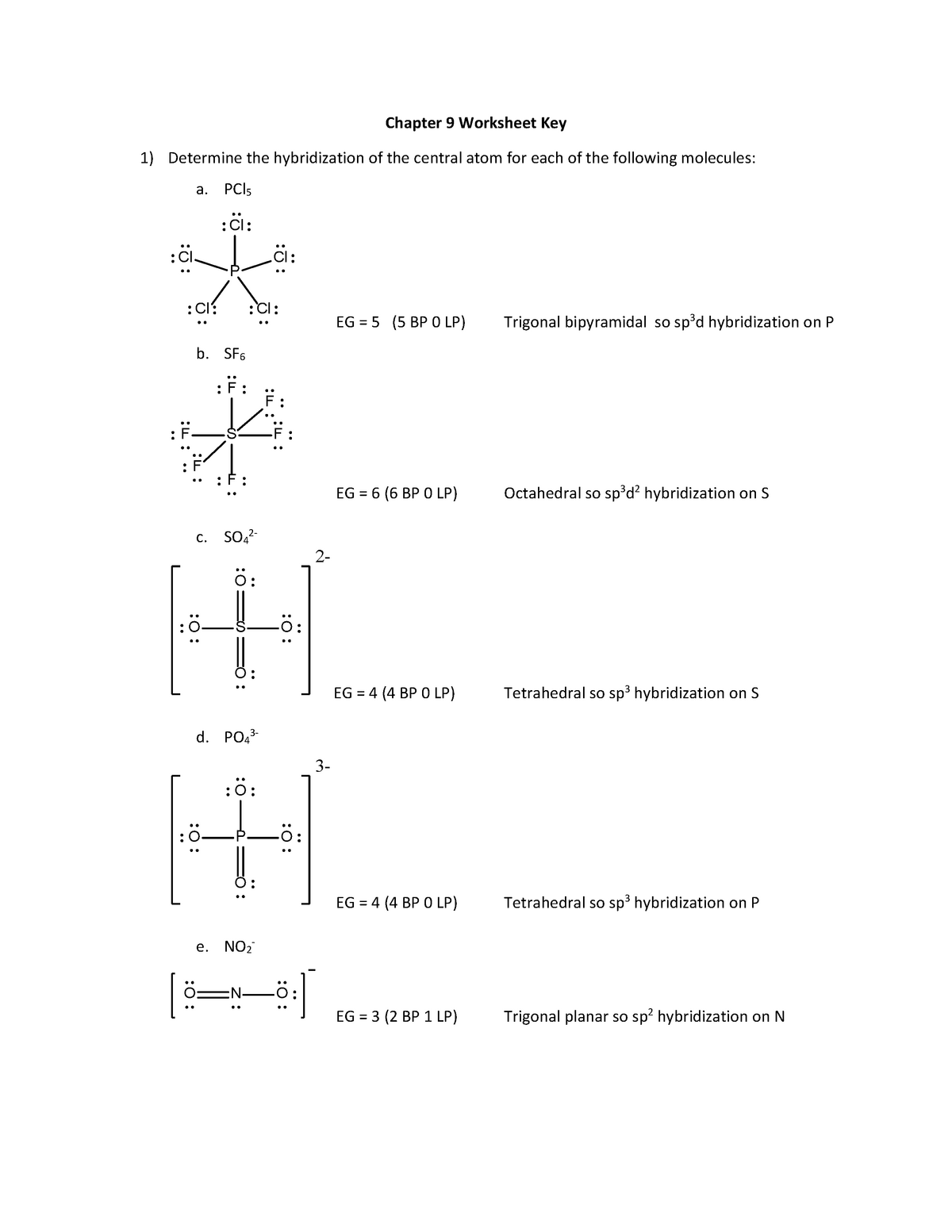
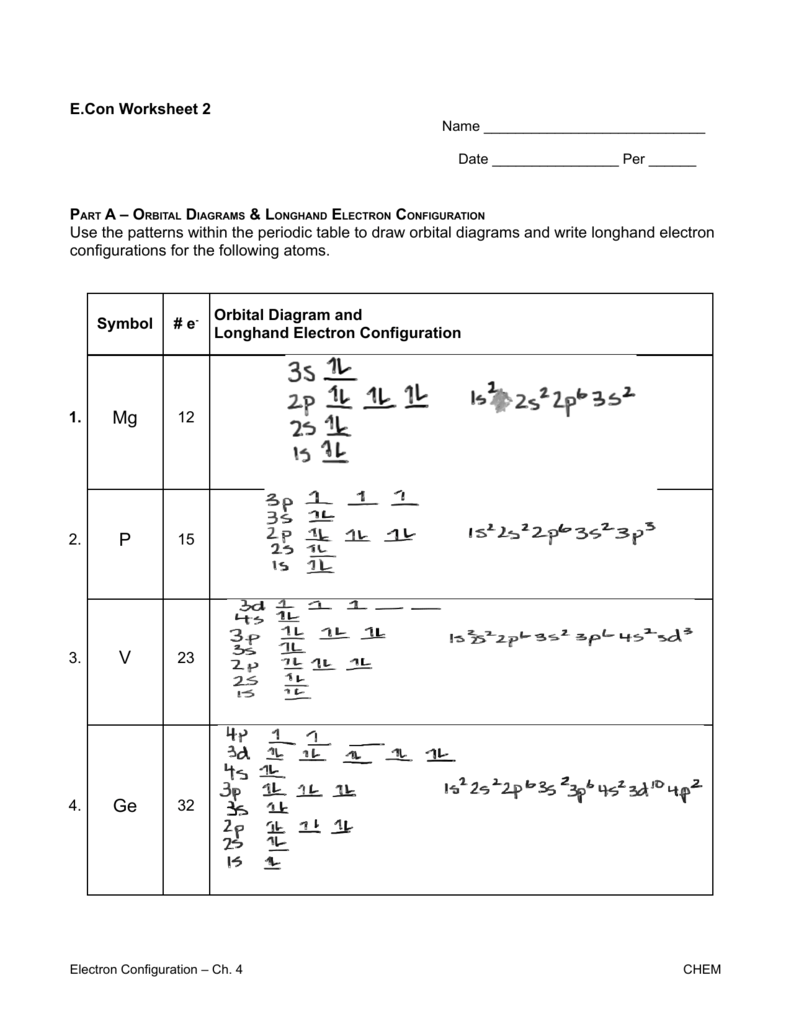
Part A - Orbital Diagrams . Use the patterns within the periodic table to draw orbital diagrams. Symbol Orbital Diagram (Don't do Nobel Gas Configuration) Mg P Ge Li Reminder of electron configuration rules: Aufbau Principle: Electrons occupy lowest energy levels first. Pauli Exculsion principle: Each orbital contains up to 2 electrons.
View Homework Help - Homework, Orbital Diagrams from CHEM 312 at Duke University. /Orbital Diagrams Name Nora- L_ Chem Worksheet 5-5 An orbital diagram uses boxes with arrows to represent the
Discussion Worksheet 11 Spring 2017 17-21. In the following problems you will be working on problems to help you understand topic 2. Use the MO diagrams provided. 17. Refer to your MO diagrams. According to molecular orbital theory, what is the bond order for each of the following: a. C22- d. Li2 18. Refer to the MO Diagrams.
The orbital filling diagram for helium. The electron configuration for helium is 1s². This means that we have two electrons in the 1s orbital, which looks like this: This diagram is exactly the same as the one for hydrogen, except that there's a second arrow added to the 1s orbital. This represents the second electron in the 1s orbital, and ...
Name : Printable Worksheets @ www.mathworksheets4kids.com Hund©s Rule & Orbital Filling Diagram Complete the orbital diagram for each element. 2) calcium 1s 2s 4s 3s 3d 2p 4p 3p 1) sodium 1s 2s 4s 3s 3d 2p 4p 3p 3) nickel 1s 2s 4s 3s 3d 2p 4p 3p 4) silicon 1s 2s 4s 3s 3d 2p 4p 3p 5) iron 6) copper 1s 2s 4s 3s 3d 2p 4p Answer key 3p 2s2 2p6 3s2 ...
2. Electron Configuration (quicker to draw than orbital filling diagrams) 2 2 Ex. O 2 1s 2s 2p4 3. Electron Dot shows only the valence (outer energy level) electrons . . Ex. :Oxygen atom . O . 1. Write orbital filling diagrams, electron configurations, and electron dot diagrams for the following elements. Table: Element Orbital Filling Diagram
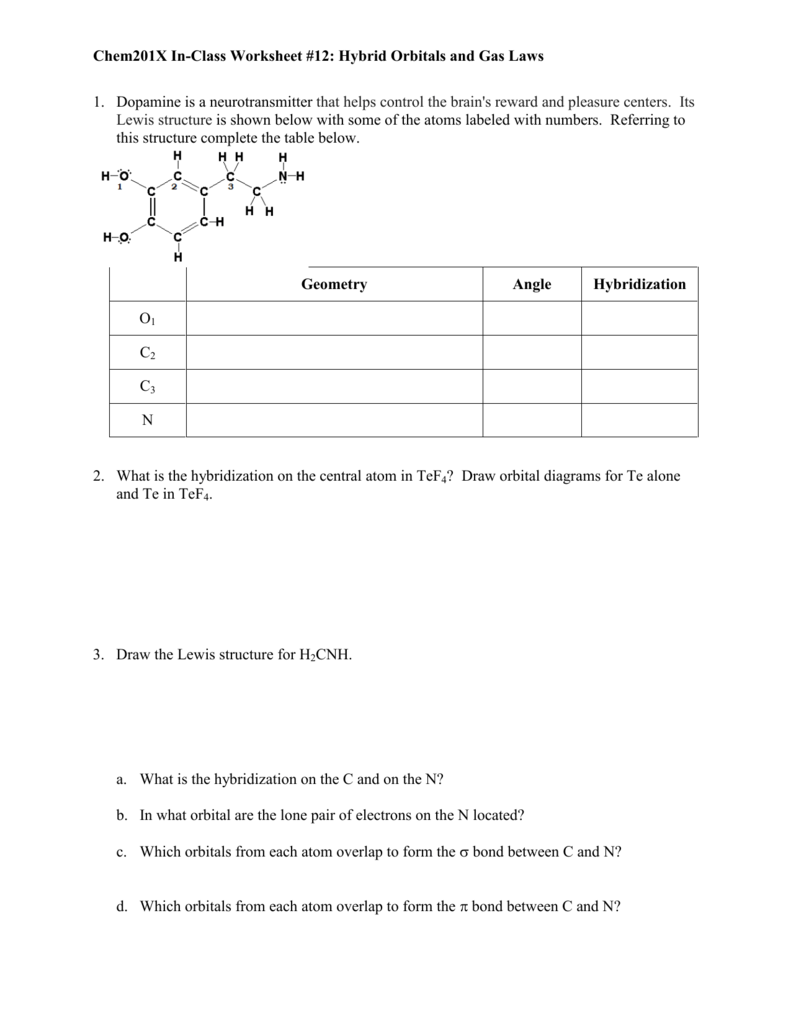
Atomic Theory and Orbitals Name Period Unit 3 Worksheet 2 Goals 1-4 1. In atoms with n 3 or larger the d orbitals can also be hybridized. Draw the shapes of each of the following orbitals. Give the n and l values for the following orbitals a. Name the orbitals described by the following quantum number.
Electron configurations orbital notation key electron. If your periodic table doesnt agree with this your answers for elements near the f. The easiest and most reliable technique for writing electron configurations is to use the periodic table as your guide. The 6 key answers for the electron configuration chem worksheet 5 are.
Use the orbital filling diagrams to complete the table. Is 2s lectron Is 4s on 2s a o o gurations or ome Orbital filling elected ements Electron 3s configuration Isl C] element (answer) en on Element O Ne 2Px 2py 2pz 2. Which element has the following orbital diagram? 3. Using arrows, show how the following orbitals will fill with electrons.
calcium has 20 electrons. No matter what the atom is, the orbital structure is the same. In class we will learn how to use the periodic table to remember the orbital structure, and then write it using the shorthand notation of electron configurations. Some things to remember: • Each orbital can contain 0, 1, or 2 electrons (and no more!).
The energy diagram for this process is shown below. The hybridized orbitals are higher in energy than the s orbital, but lower in energy than the p orbitals. atomic orbitals hybridized orbitals Carbon has 4 valence electrons. Add these electrons to the atomic and molecular orbitals. This hybridization gives tetrahedral geometry.
-Orbital diagrams are visual representations of electron configuration . Hund's Rule •When electrons are filling orbitals of the same energy, they prefer to enter empty orbitals first. These electrons all have the same spin •A diagram of nitrogen is shown below (7 total
Homework Worksheet #3: Electron Configurations ... Orbital Diagram Configuration Orbital Diagram Configuration . Dr. Mihelcic Honors Chemistry 2013-14 8 Homework Worksheet #7: Electron Configurations ... your answer for each. Hund's Rule, Pauli Exclusion Principle, Aufbau Principle ...
























Comments
Post a Comment