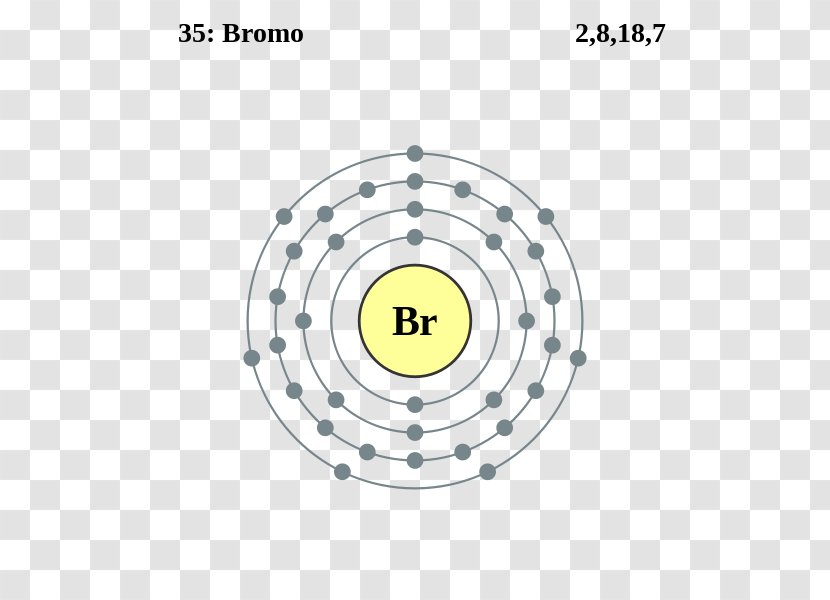
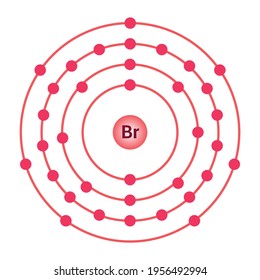
39 bromine bohr diagram
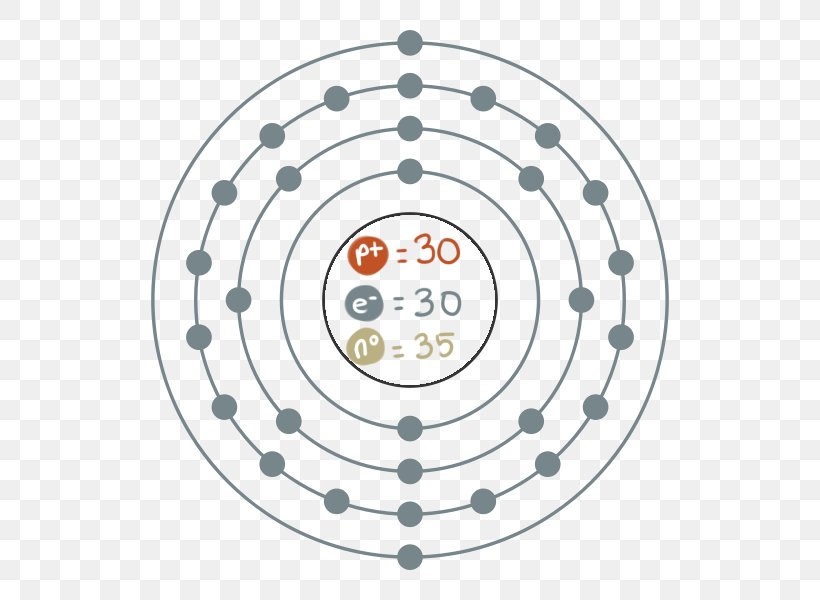
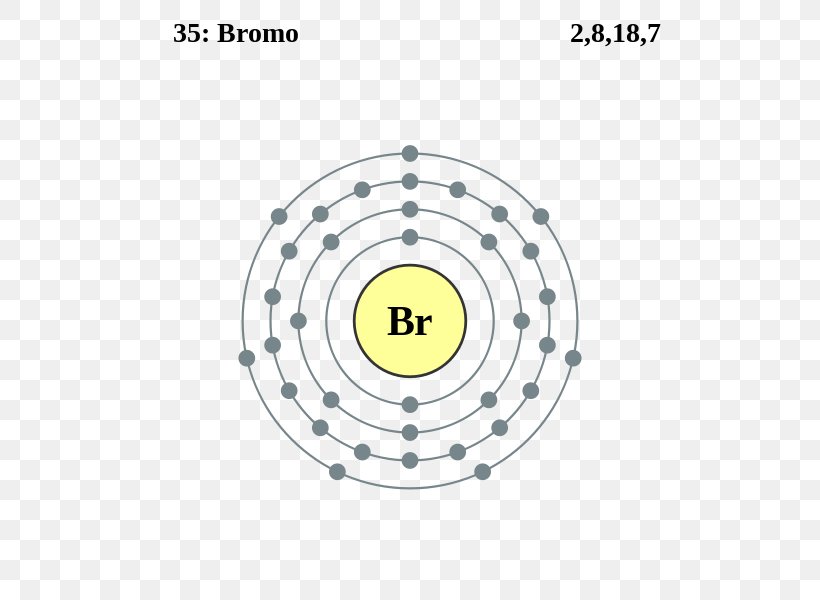
Build An Atom - HTML5 published by the PhET This is the newer HTML5 version of PhETs popular Build An Atom interactive simulation. The ground state electron configuration is the lowest energy combination of electrons in the atomic orbitals. The properties of the assorted elements are dependent on the selection of protons and electrons they have. Diagram of the nuclear composition and electron configuration of an atom of bromine-79 (atomic number: 35), the most common isotope of this element. The nucleus ...
Steps to draw the lewis dot structure of BrCN. In cyanogen bromide, a carbon atom forms a triple bond with a nitrogen atom (cyanogen group) and a single covalent bond with bromine, a halogen. Step 1: First, the skeleton structure of the compound must be drawn, using the symbol of the elements and joining them by single bonds only.

Bromine bohr diagram
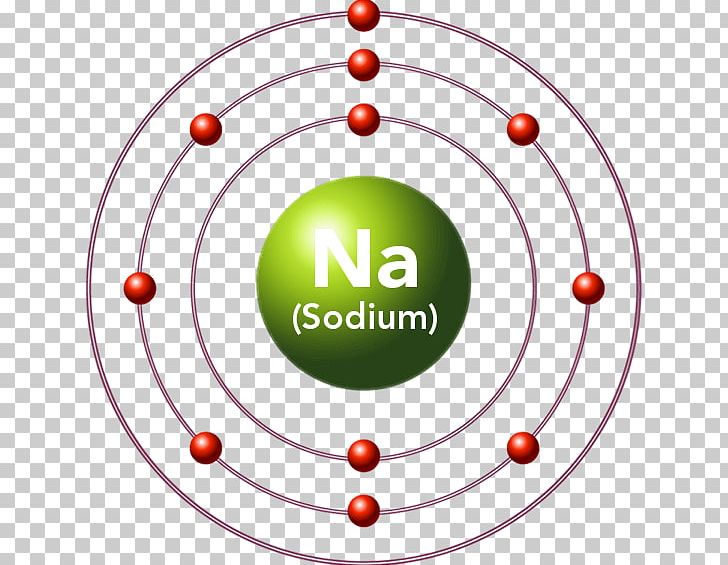
Bohr model of Beryllium (Be) 2, 2: 5: Bohr model of Boron (B) 2, 3: 6: Bohr model of Carbon (C) 2, 4: 7: Bohr model of Nitrogen (N) 2, 5: 8: Bohr model of Oxygen (O) 2, 6: 9: Bohr model of Fluorine (F) 2, 7: 10: Bohr model of Neon (Ne) 2, 8: 11: Bohr model of Sodium (Na) 2, 8, 1: 12: Bohr model of Magnesium (Mg) 2, 8, 2: 13: Bohr model of ... Chem4Kids.com! Bromine atomic orbital and chemical bonding information. There are also tutorials on the first thirty-six elements of the periodic table. An orbital is the quantum mechanical refinement of Bohr's orbit. ... Figure \(\PageIndex{3}\) The diagram of an electron configuration . specifies the subshell and superscript number of electrons. Table \(\PageIndex{2}\) shows the electron configurations of the elements in the first and second periods.
Bromine bohr diagram. This is a Lewis dot diagram. To work out the valence level for Lewis dot diagrams, remember from Organics 1 Lesson 3: Step 1 - Determine the total valence electrons for the atom (use energy level/Bohr diagram OR group numbers on periodic table). Step 2 - Use the element symbol to represent the nucleus and the inner filled electron energy levels. Find bromine atom stock images in HD and millions of other royalty-free stock photos, ... bohr model of the bromine atom. electron structure of bromine. INTERNET CITY, DUBAI, Nov. 23, 2021 - LBank Exchange, a global digital asset trading platform, has listed BoHr (BR) token on November 22, 2021. For all users of LBank Exchange, the BR/USDT ... Basic atomic structure worksheet answers 1 a protons b neutrons c electrons a positive b neutral c negative 2 atomic number or identity. Electrons in a neutral charge atom only. Mass 5 mass number. The number of protons in one atom of an element determines the atom s number of electrons determines the of the elemen in one atom of an element.
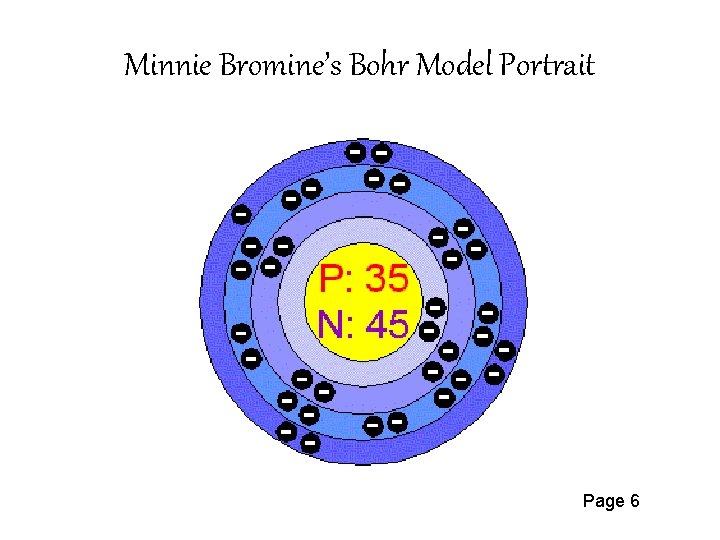
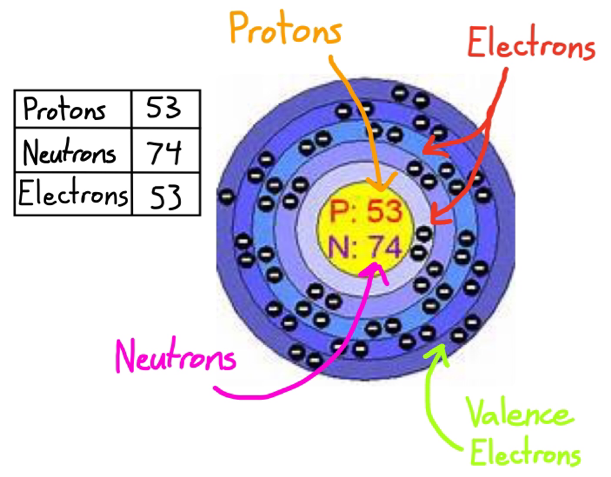
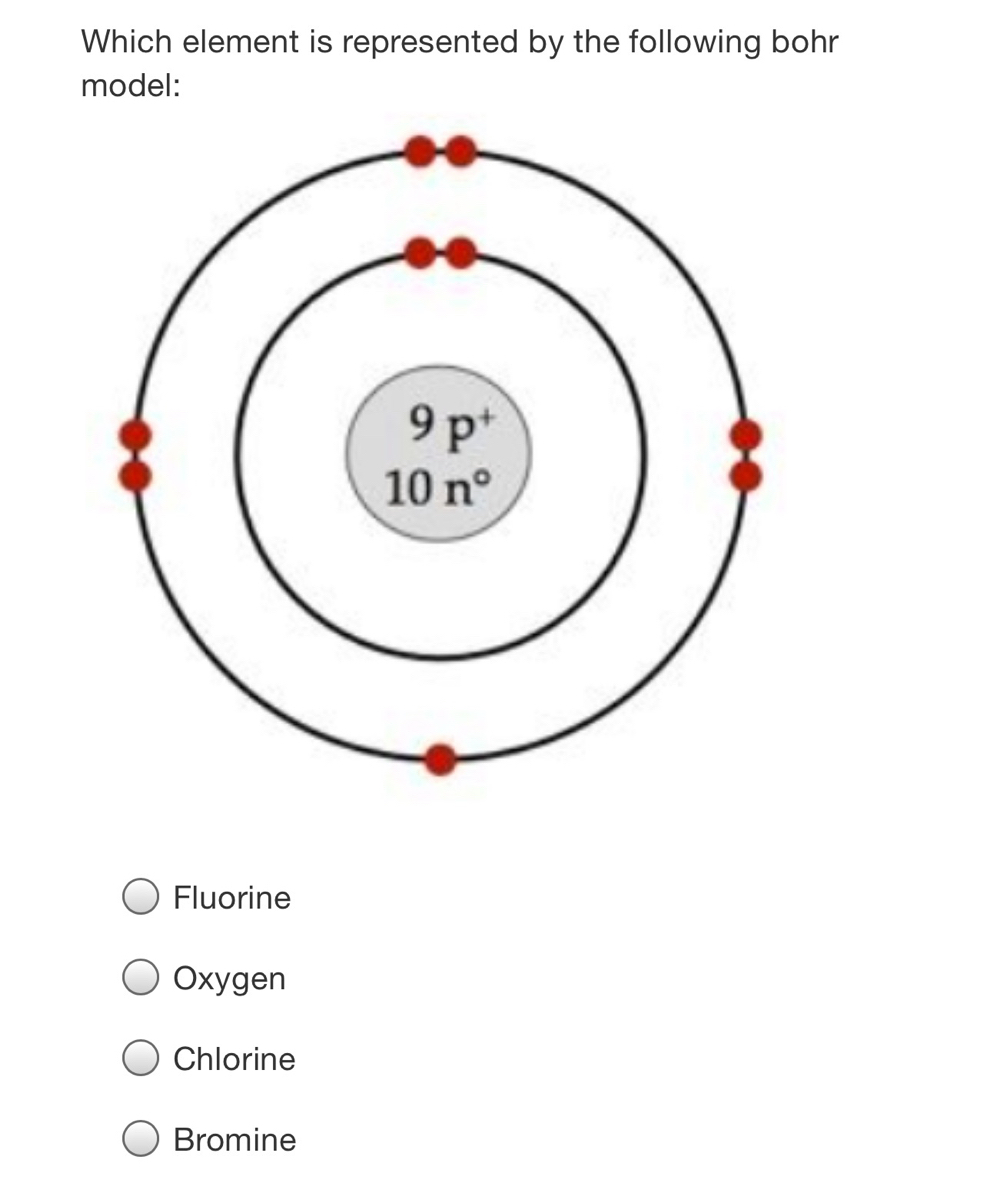
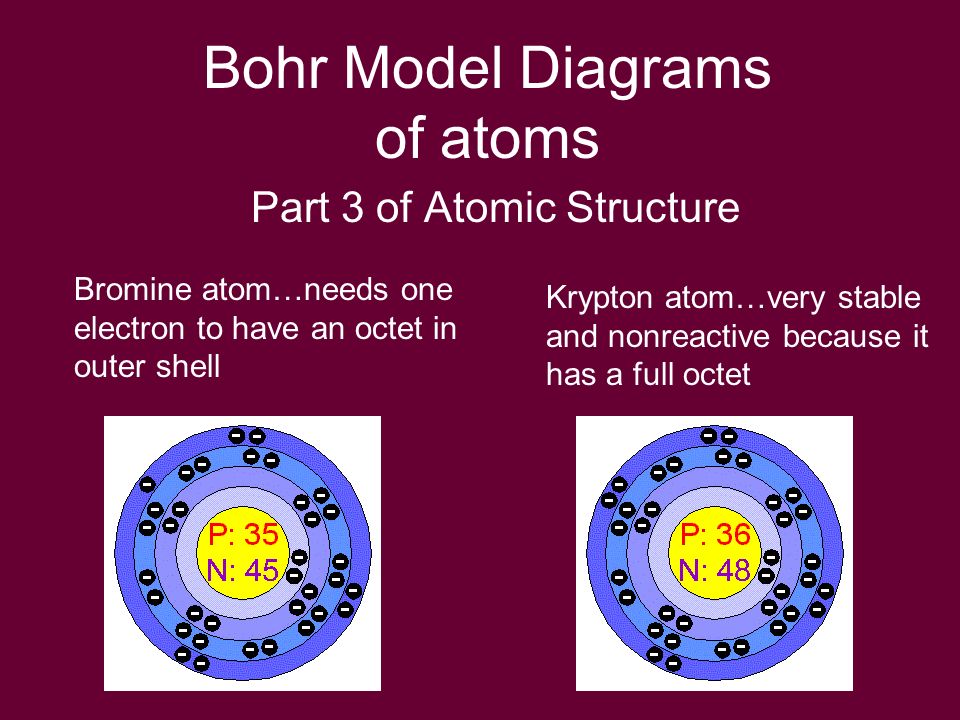
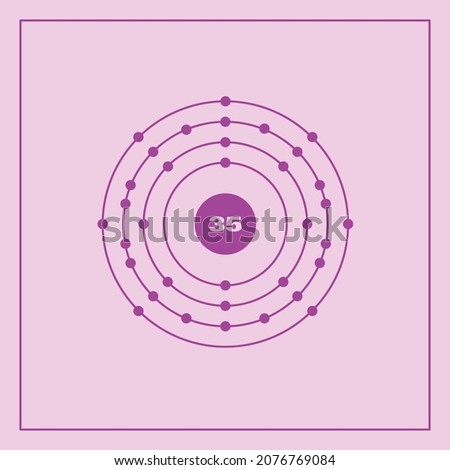
Describe Bohr's model of the atom. Answer: A Danish physicist, Neils Bohr proposed an atomic model in 1913. This model of atom is called Bohr's model of atom. Basic postulates of the Bohr's atomic model are : (i) In an atom, the electrons revolve around the nucleus in certain definite circular orbits. Draw the Lewis diagram for each compound. a molecule composed of two chlorine atoms; a molecule composed of a hydrogen atom and a bromine atom; Solution. Chlorine has the same valence shell electron configuration as fluorine, so the Lewis diagram for a molecule composed of two chlorine atoms is similar to the one for fluorine: The Bohr model of Bromine(Br) is drawn with four electron shells, the first shell contains 2 electrons, the second shell contains 8 electrons, the third shell ...Steps to draw the Bohr Model... · Find Valence electron of... 35 Bohr Model of Bromine Number of EnergyIn atomic physics the RutherfordBohr model or Bohr model or Bohr diagram presented by Niels Bohr and Ernest Rutherford in a system consisting of a small dense nucleus surrounded by revolving electrons. These are the only two natural isotopes with 79Br making up 51 of natural bromine.
Aug 15, 2020 — Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are ... Bohr's model of the atom: Niels Bohr proposed the following postulates regarding the model of the atom. Only certain orbits known as discrete orbits of electrons are allowed inside the atom. While revolving in these discrete orbits, the electrons do not radiate energy. These discrete orbits or shells are shown in the following diagram. Atomic structure refers to the structure of atom comprising a nucleus (center) in which the protons (positively charged) and neutrons (neutral) are present. In the reaction between bromine and potassium iodide, there is a reduction of bromine to bromide ions. 2012-09-19 23:26:49 2012-09-19 23:26:49. Internet City, Dubai--(Newsfile Corp. - November 25, 2021) - LBank Exchange, a global digital asset trading platform, has listed BoHr (BR) token on November 22, 2021. For all users of LBank ...
The bromine has 7 valence electrons; the 4s orbital will naturally be occupied by 2 electrons. The remaining five electrons will go and occupy the 4p orbital. Hence the full Ground state electronic configuration for bromine in accordance with the Aufbau Principle is: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5.
What is an electron orbital diagram? An orbital diagram , or orbital box diagram , is a way of representing the electron configuration of an atom. A box, line, or circle, is drawn to represent each orbital in the electron configuration . (using the Aufbau Principle to order the orbitals and hence the boxes, lines or circles, as shown below) 1s.
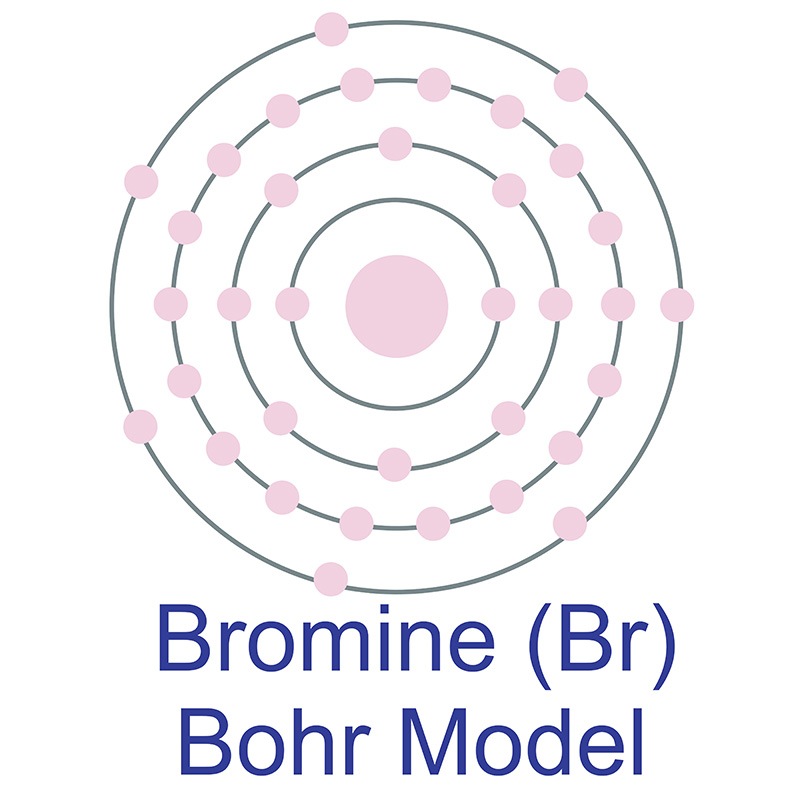
Name: Bromine Symbol: Br Atomic Number: 35. Atomic Mass: 79.904 amu ... [Bohr Model of Bromine], Number of Energy Levels: 4. First Energy Level: 2
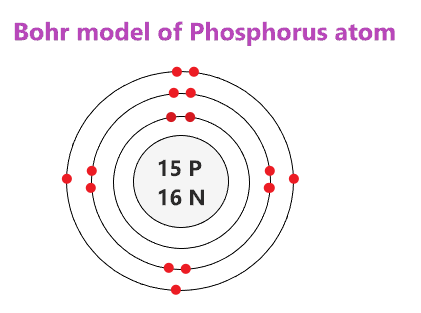
Bohr Diagram Of Sodium Atom Diagram Atom Model Atom Model Project . Image Result For Aluminum Atom Model 8th Grade Science Montessori Science Atom . 15 P Phosphorus Electron Shell Structure Schoolmykids Element Chemistry Atomic Structure Shell Structure . File Electron Shell 035 Bromine Svg Wikimedia Commons Science Education Electrons Shells .

Electron Configuration Bohr Model Atom Chemical Element Png Clipart Argon Atom Atomic Orbital Body Jewelry Bohr Model Free Png Download
We break down the anatomy of these structures to display this for students and we will explore the bohr model of this structure. Mass number 7 lithium li 3 bromine br 35 iron fe 26 copper cu 29 oxygen o 8 mercury hg 80 krypton kr 36 helium he 2 8 a neutral atom by definition has no charge.

See The Electron Configuration Diagrams For Atoms Of The Elements Electron Configuration Atom Diagram Electrons
Jun 14, 2016 — 35_bromine_(Br)_enhanced_Bohr_model.png (466 × 471 pixels, file size: 41 KB, MIME type: image/png). File information. Structured data ...
Out that these four quantum numbers, however, Bohr postulated that just the major quantum number, n, determines the energy of the electron. Therefore, the 3s orbital (l=0) has actually the same energy as the 3p (l=1) and 3d (l=2) orbitals, regardless of a difference in l values. This postulate, however, holds true only for Bohr"s hydrogen atom ...
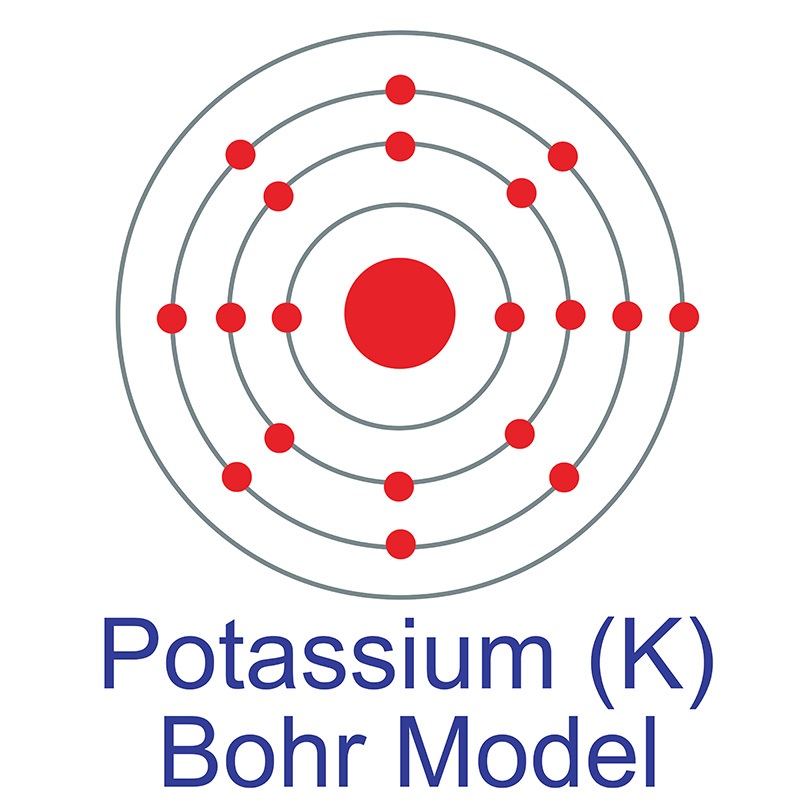
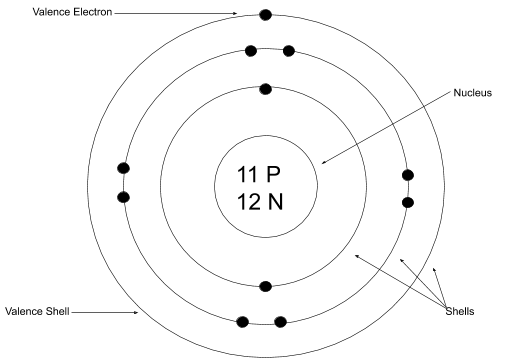
Electronic Structure. Electrons are found in energy levels (shells). There is a maximum of two electrons in the innermost shell, up to eight in the second shell and up to eight in the third shell. For example, a sodium atom has an electronic structure of 2, 8, 1.
10. If bromine atom is available in the form of, say, two isotopes 79/35 Br (49.7%) and 81/35 Br (50.3%), calculate the average atomic mass of bromine atom. Answer It is given that two isotopes of bromine are 79/35 Br (49.7%) and 81/35 Br (50.3%). Then, the average atomic mass of bromine atom is given by: NCERT SOLUTION: Structure of the Atom. 11.

Bromine Br On Twitter This Is The Bohr Model For Bromine Element That Shows The 35 Protons Electrons And 45 Neutrons It Also Shows The Number Of Valence Electrons Which Is 7 The
Electrons arrangement in Bromine or Bohr model of Bromine: 2, 8, 18, 7: Electronic configuration of Bromine [Ar] 3d 10 4s 2 4p 5: Atomic radius of Bromine: 183 picometers (van der Waals radius) Valence electrons in Bromine: 5: 1st Ionization energy of Bromine: 13.61 eV: Electronegativity of Bromine: 2.96 (Pauling scale) Crystal structure of ...
The average radius for bromine is 115 pm, its atomic radius or Bohr radius is 94 pm, its covalent radius is 114 pm, and its Van der Waals radius is 185 pm. Bromine has a total of 35 electrons whose distribution is as follows: In the first layer it has 2 electrons, in the second it has 8 electrons, in its third layer it has 18 electrons and in ...
Jul 13, 2018 — The bohr model shows the atom as a central nucleus containing protons and neutrons with the electrons in circular orbitals at specific distances ...

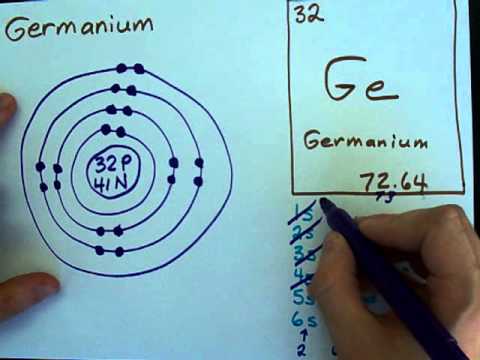
Electron Configuration Germanium Electron Shell Bohr Model Valence Electron Color Table Chemical Element Electron Png Pngegg
If bromine atom is available in the form of say, two isotopes 35 Br 79 (49.7%) and 35 Br 81 (50.3%). Calculate the average atomic mass of bromine atom. Calculate the average atomic mass of bromine atom.
Magnesium Chemical Element Bohr Model Diagram Png Clipart Area Atom Bohr Model Chemical Element Chemistry Free
An orbital is the quantum mechanical refinement of Bohr's orbit. ... Figure \(\PageIndex{3}\) The diagram of an electron configuration . specifies the subshell and superscript number of electrons. Table \(\PageIndex{2}\) shows the electron configurations of the elements in the first and second periods.
Chem4Kids.com! Bromine atomic orbital and chemical bonding information. There are also tutorials on the first thirty-six elements of the periodic table.
Bohr model of Beryllium (Be) 2, 2: 5: Bohr model of Boron (B) 2, 3: 6: Bohr model of Carbon (C) 2, 4: 7: Bohr model of Nitrogen (N) 2, 5: 8: Bohr model of Oxygen (O) 2, 6: 9: Bohr model of Fluorine (F) 2, 7: 10: Bohr model of Neon (Ne) 2, 8: 11: Bohr model of Sodium (Na) 2, 8, 1: 12: Bohr model of Magnesium (Mg) 2, 8, 2: 13: Bohr model of ...

Br Bromine Element Information Facts Properties Trends Uses And Comparison Periodic Table Of The Elements Schoolmykids




:max_bytes(150000):strip_icc()/Selenium-58b601fd3df78cdcd83d2a90.jpg)
:max_bytes(150000):strip_icc()/Silver-58b601c15f9b5860464c0e8f.jpg)





Comments
Post a Comment