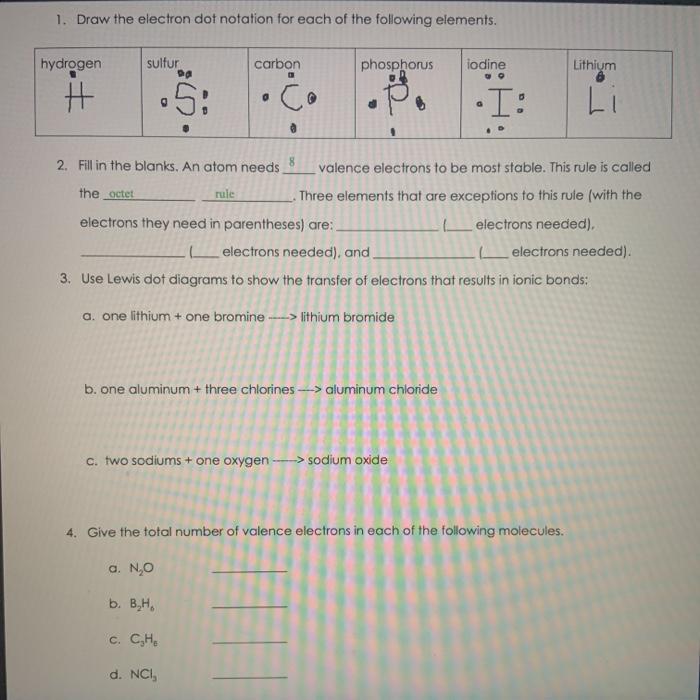
39 electron dot diagram for phosphorus
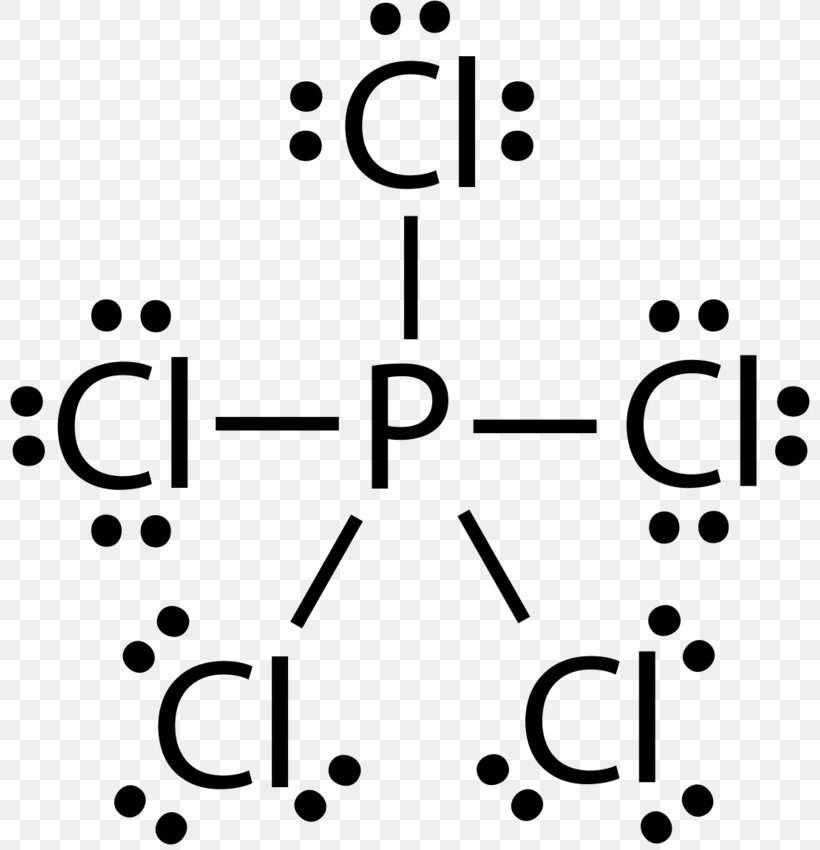
PF3 lewis structure, Molecular geometry, Polar or nonpolar ... The molecular geometry or shape for PF3 is the trigonal pyramid. The electron geometry for PF3 is tetrahedral as it central has 4 regions of electron density. Lewis dot structure of PF3 contains 1 lone pair on the central atom (phosphorous) and 3 lone pairs on each outer atom (fluorine). Subscribe to Blog via Email. Lewis Dot Diagram Phosphorus - schematron.org Sep 23, 2018 · Draw a Lewis electron dot diagram for an atom or a monatomic ion. In almost all cases, chemical The electron dot diagram for helium, with two valence electrons, is as follows: By putting the two phosphorus; argon. Answer. For atoms with. Phosphorus has 5 valence electrons.
What is the Lewis electron dot diagram for phosphorus? Jun 6, 2021 — So, to draw the Lewis Structure, begin by drawing the symbol for Phosphorus, the letter P. Next, Phosphorus has 5 valence electrons.
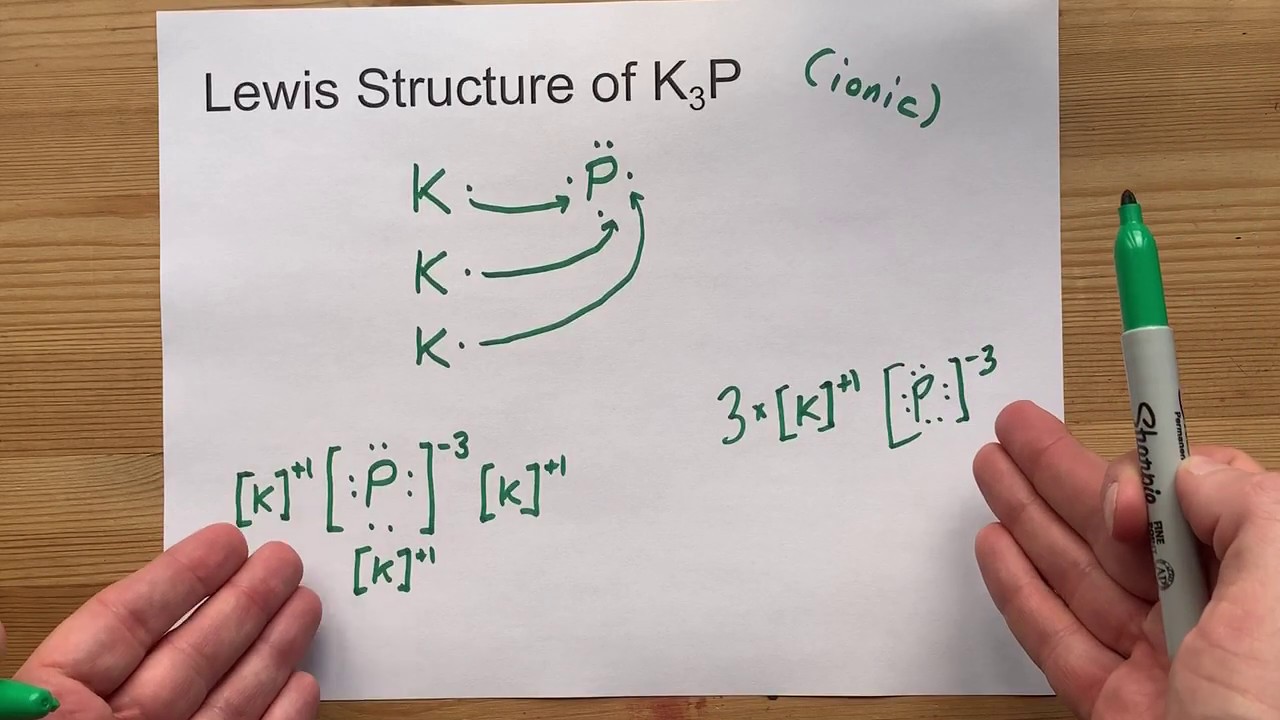
Electron dot diagram for phosphorus
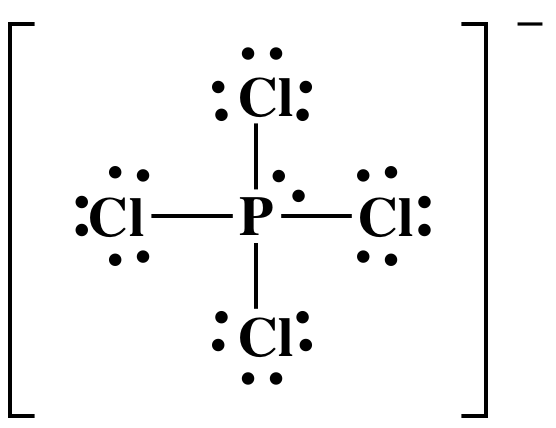
Lewis Dot Diagram For Phosphorus - schematron.org Dec 26, 2018 · The DOT ID is also the UN Number">. More information about the DOT ID/UN number and the guide number can be found at the Emergency Response Guidebook. Nov 22, · Best Answer: The Lewis Electron Dot Diagram for Phosphorous is P with 5 dots around it. There are 5 valence electrons, so this is how many dots are around. Each dot represents one electron. 6.1 Lewis Electron Dot Diagrams | Introductory Chemistry Draw the Lewis electron dot diagram for each element. a) titanium. b) phosphorus. 8. Draw the Lewis electron dot diagram for each element. a) bromine. b) ... PO43- Lewis Structure - Learnool Mar 02, 2022 · Valence electrons of one phosphorus atom = 5 × 1 = 5. Valence electrons of four oxygen atoms = 6 × 4 = 24. Now the PO 43- has a negative (-3) charge, so we have to add three more electrons. So the total valence electrons = 5 + 24 + 3 = 32. Second, find the total electron pairs. We have a total of 32 valence electrons.
Electron dot diagram for phosphorus. 9.1 Lewis Electron Dot Diagrams What is the Lewis electron dot diagram for each element? phosphorus; argon. Answer. For atoms with partially filled d or f subshells, these electrons are ... Electron Configuration for Phosphorus (P) - UMD The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next two electrons in the 3s. Since the 3s if now full we'll move to the 3p where we'll place the remaining three electrons. Therefore the Phosphorus electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 3. Video: Phosphorus Electron Configuration Notation PO43- Lewis Structure - Learnool Mar 02, 2022 · Valence electrons of one phosphorus atom = 5 × 1 = 5. Valence electrons of four oxygen atoms = 6 × 4 = 24. Now the PO 43- has a negative (-3) charge, so we have to add three more electrons. So the total valence electrons = 5 + 24 + 3 = 32. Second, find the total electron pairs. We have a total of 32 valence electrons. 6.1 Lewis Electron Dot Diagrams | Introductory Chemistry Draw the Lewis electron dot diagram for each element. a) titanium. b) phosphorus. 8. Draw the Lewis electron dot diagram for each element. a) bromine. b) ...
Lewis Dot Diagram For Phosphorus - schematron.org Dec 26, 2018 · The DOT ID is also the UN Number">. More information about the DOT ID/UN number and the guide number can be found at the Emergency Response Guidebook. Nov 22, · Best Answer: The Lewis Electron Dot Diagram for Phosphorous is P with 5 dots around it. There are 5 valence electrons, so this is how many dots are around. Each dot represents one electron.




/Lewis-dot-58f78f405f9b581d5938e617.jpg)

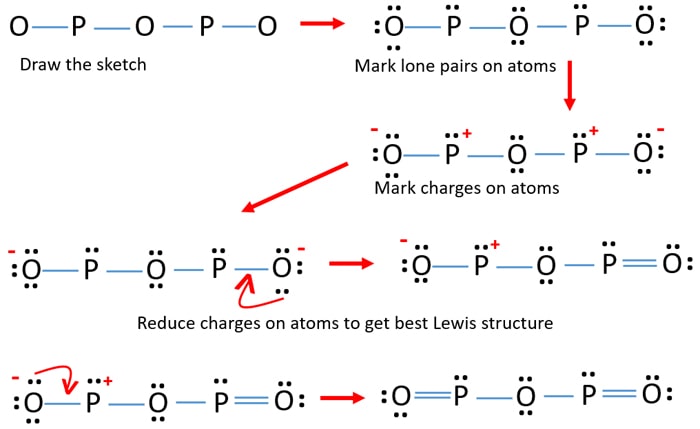

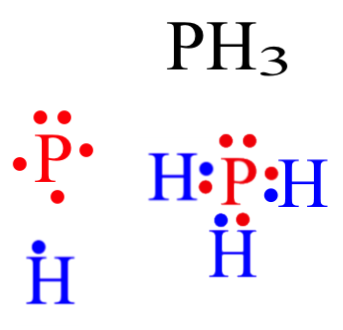
/Lewis-dot-58f78f405f9b581d5938e617.jpg)

















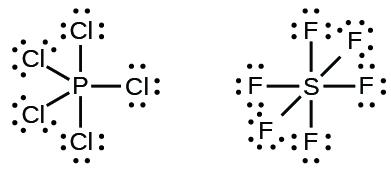
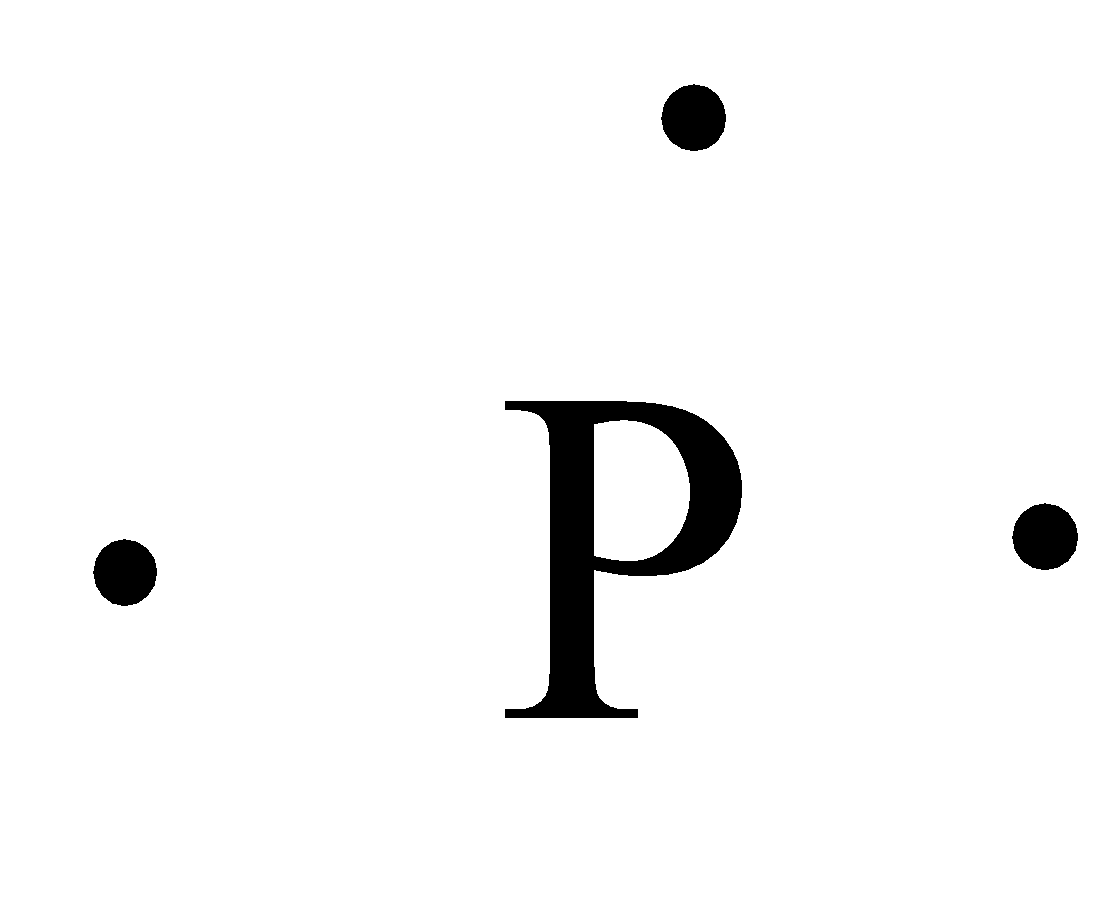


Comments
Post a Comment