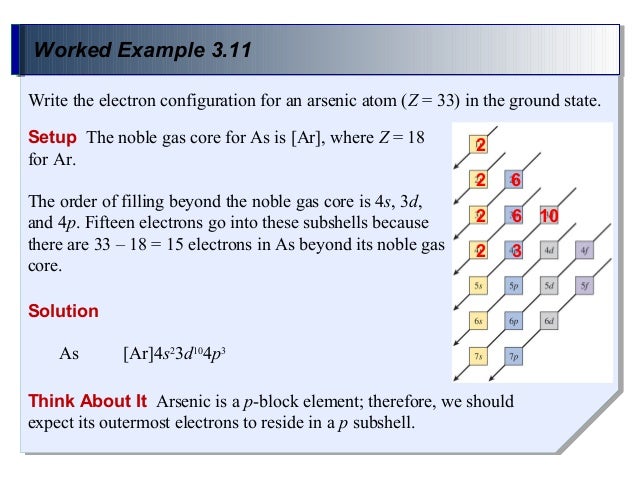
39 atomic orbital diagram for arsenic
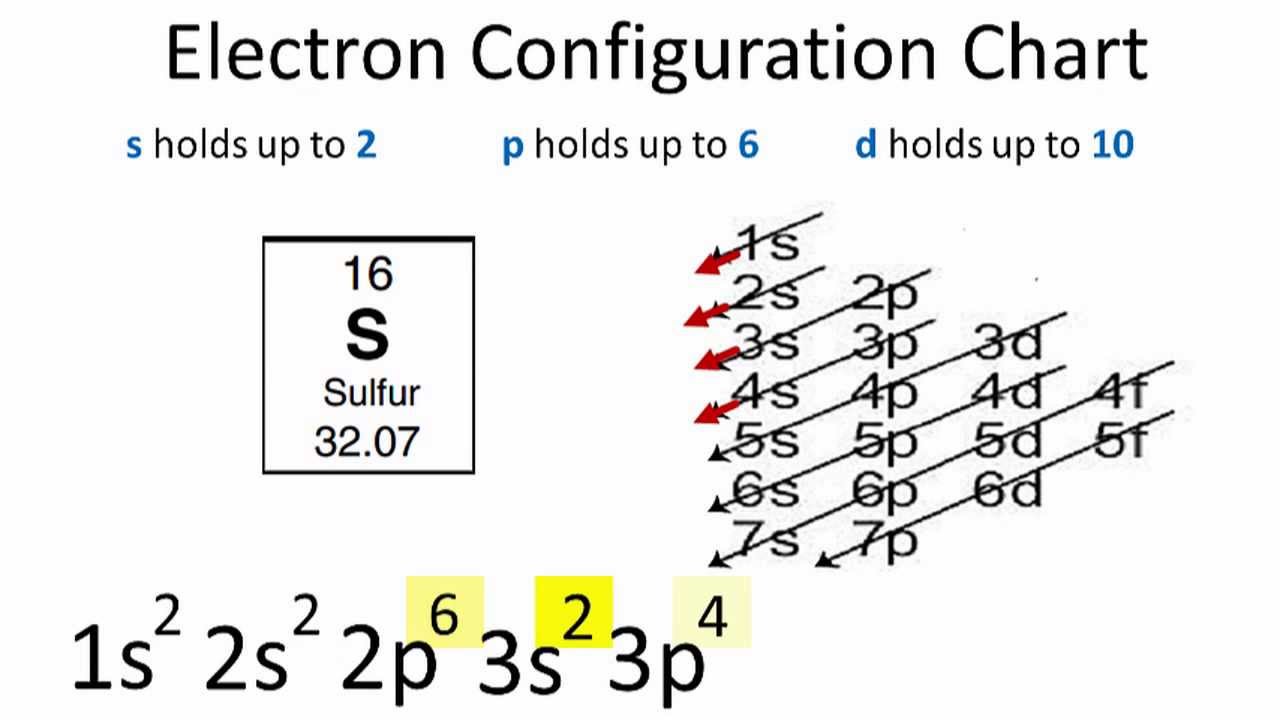
Our arsenic page has over 190 facts that span 107 different quantities. ... [Ar] represents the closed-shell electron configuration of argon ... The orbital notation of arsenic (As) is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3See the Related Questions for the electron configuration of all the elements. What is the orbital diagram for bromine? The...
Electron Orbital Notation for Arsenic. Mr. Causey shows you step by step how to write the electron configuration and the orbital notation ...

Atomic orbital diagram for arsenic
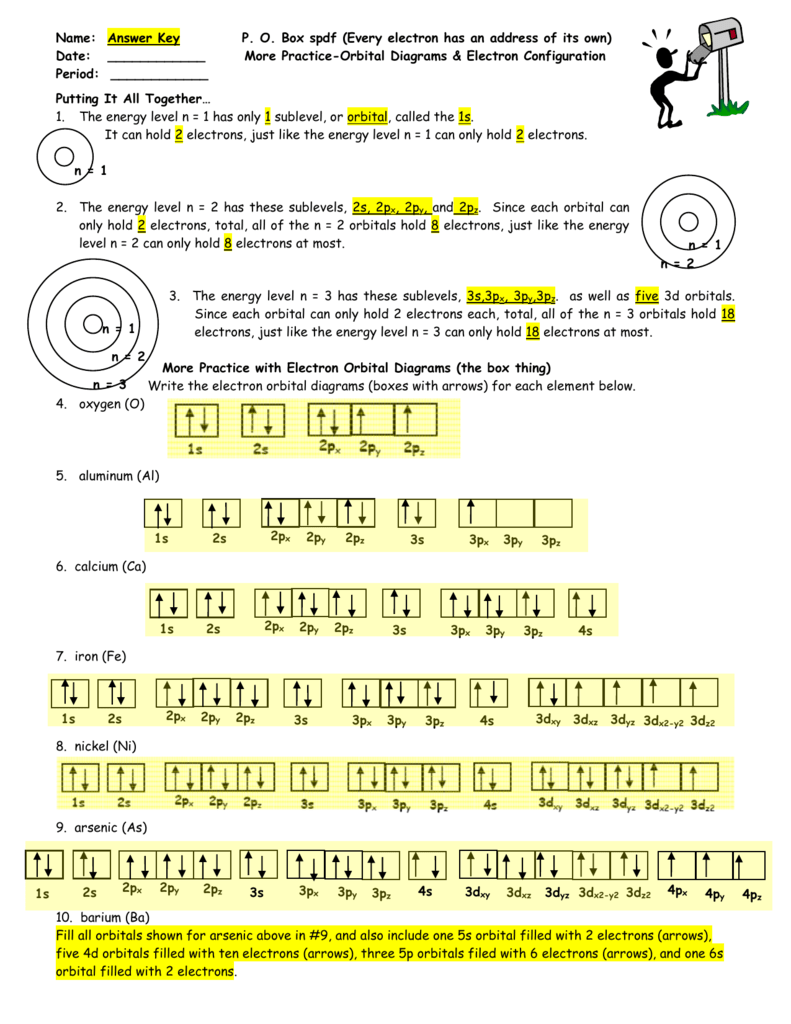
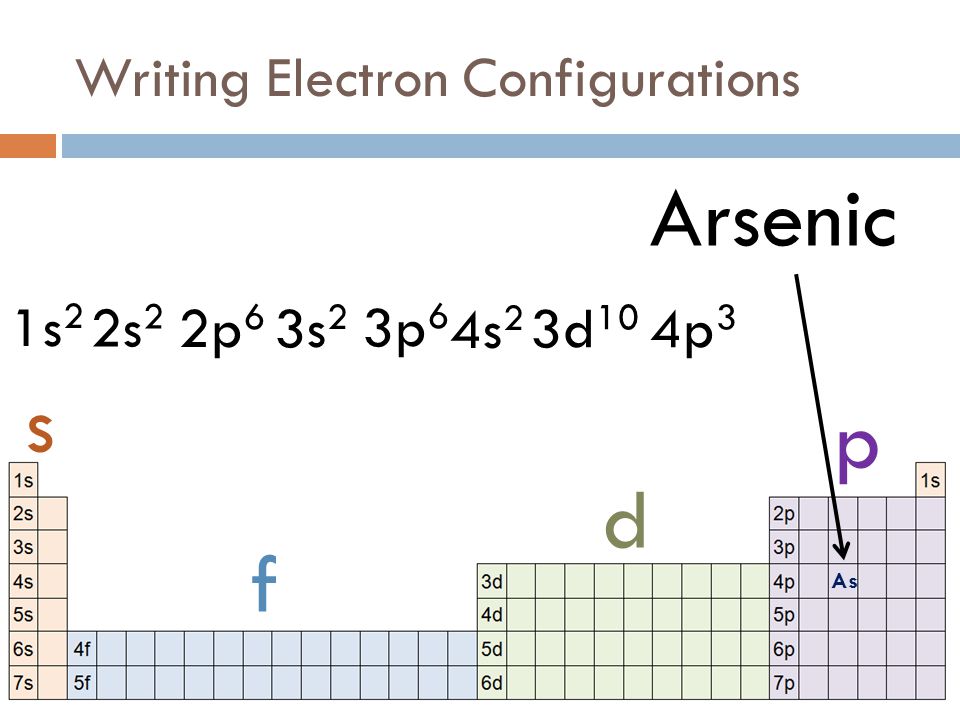
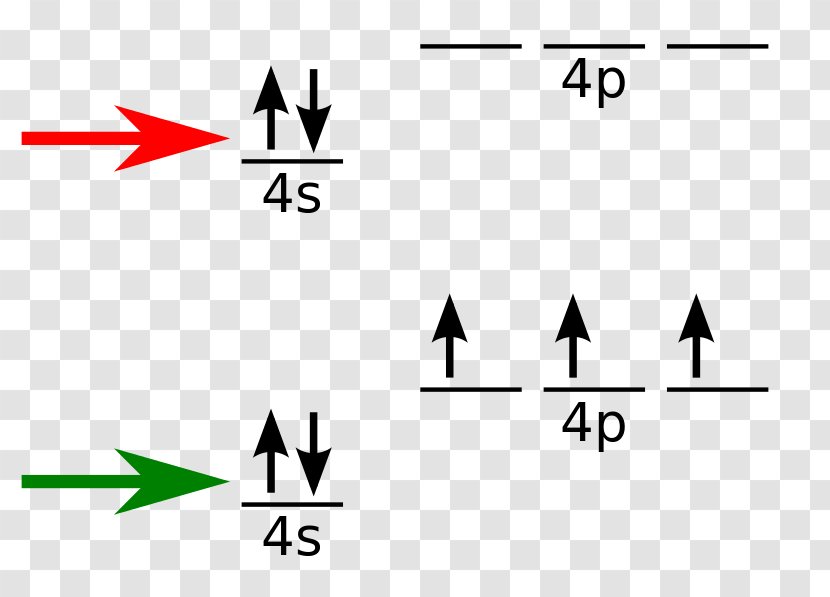
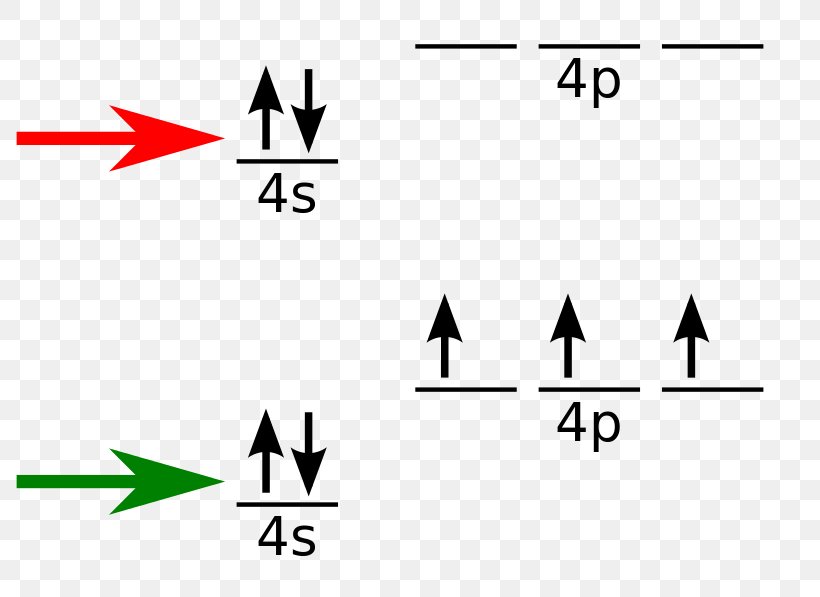
What is the orbital notation of arsenic? The orbital notation of arsenic (As) is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3See the Related Questions for the electron configuration of all the elements. What... Because the 4p section has 3 orbitals, but Arsenic ends with 4p3. It'll want to leave as few orbitals empty, so you have three arrows pointing up. The orbital diagram of arsenic can be written as 1s2 2s2 2p6 3s23p6 4s2 3d10 4p3. Arsenic has 33 electrons, including 3 in itsoutermost shell. schematron.org! Arsenic atoms have 33 electrons and the shell structure is 2.8.18.5. The ground state electron configuration of ground state gaseous neutral arsenic is [Ar].3d ...
Atomic orbital diagram for arsenic. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ... These orbital designations are derived from corresponding spectroscopic characteristics of lines involving them: s harp, p rinciple, d iffuse, and f undamental. Orbital diagram for arsenic Flickr user Jonathan Quintana loves to customize desktops, and this Ubuntu setup is his first linux desktop. It looks sharp, offers some useful information, and still has plenty of room to work and do things. Jan 24, 2022 · Arsenic Electron Configuration - 9 images - rubidium electron configuration rb with orbital diagram, arsenic properties and occurrence assignment point,
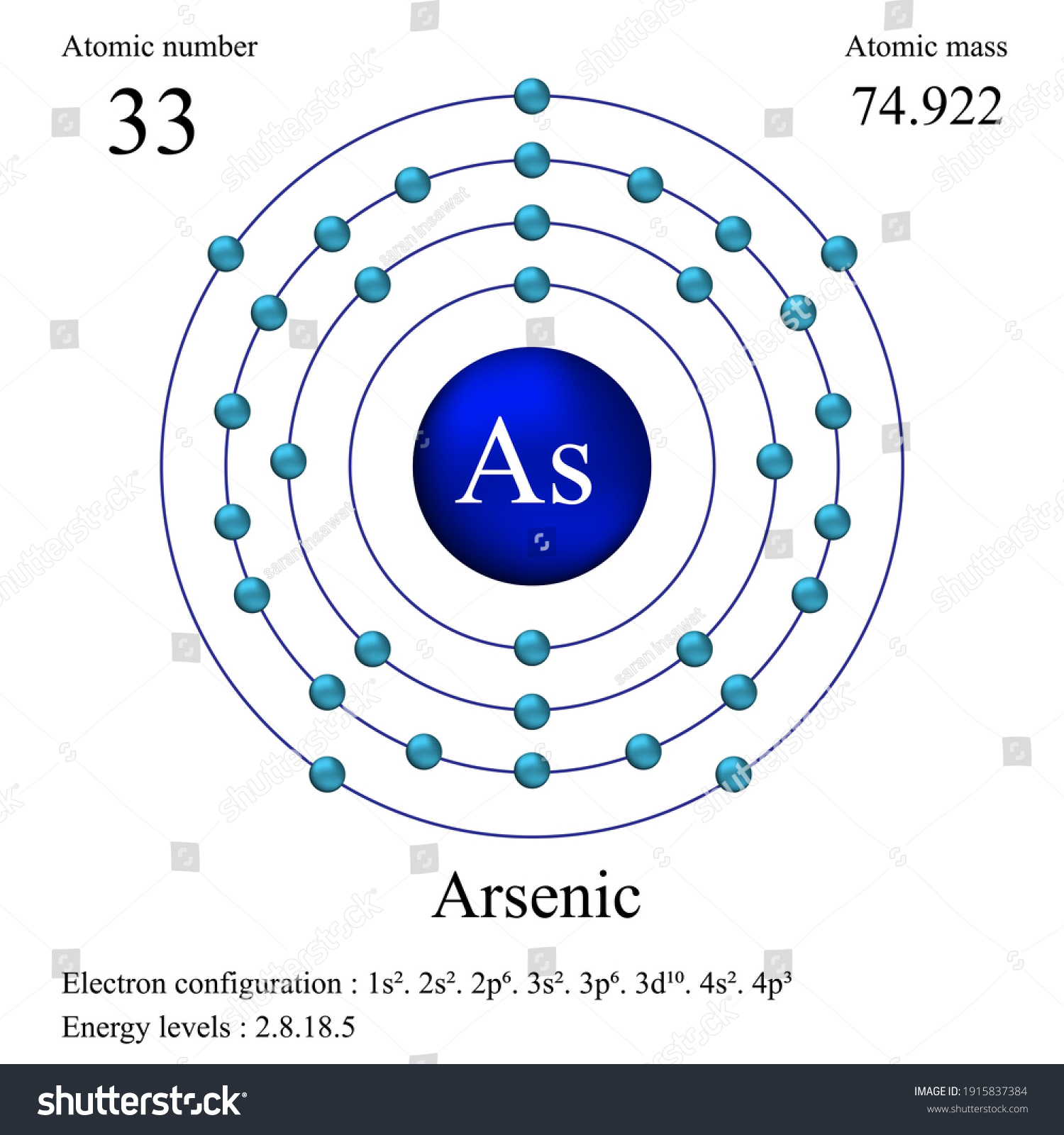
Arsenic electron configuration ; Electronic configuration of the Arsenic atom in ascending order of orbital energies: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p ; Electronic ... Arsenic (As) Atomic Data for Arsenic (As) Atomic Number = 33 Atomic Weight = 74.9216 Reference E95 As I Ground State 1s22s22p63s23p63d104s24p34S°3/2 Ionization energy 78950 cm-1(9.7886 eV) Ref. BJ71 As II Ground State 1s22s22p63s23p63d104s24p23P0 Ionization energy 149932 cm-1(18.5892 eV) Ref. LA71 By using the Aufbau diagram (shown below) we can determine the full electron configuration for a neutral arsenic atom. 1s22s22p63s23p63d104s24p3 The shorthand electron configuration, which uses the symbol for the noble gas in the previous period, in this case argon. [Ar]3d104s24p3 Aufbau Diagram Answer link To write the orbital diagram for the Arsenic atom (As) first we need to write the electron configuration for just As. To do that we need to find the number o...
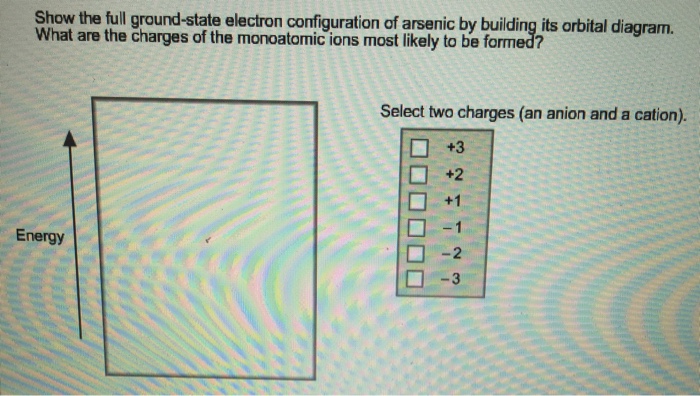
Scientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the orbit is given there. The electrons of the atom revolve around the nucleus in a certain circular path. These circular paths are called orbit(shell). These orbits are expressed by n. [n = 1,2,3,4 . . . The serial number of the orbit] K is the name of the first orbit, L is the second, M is the third, N is the name of the fourth orbit. The electron holding capacity of each orbit is 2n2. For example, 1. n = 1 for K orbit. The electron holding capacity of K orbit is 2n2 = 2 × 12= 2 electrons. 2. For L orbit, n = 2. The electron holding capacity of the L orbit is 2n2 = 2 × 22= 8 electrons. 3. n=3 for M orbit. The maximum electron holding capacity in M orbit is 2n2 = 2 × 32 = 18 electrons. 4. n=4 for N orbit. The maximum electron holding capacity in N orbit is 2n2 = 2 × 42= 32 electrons. Therefore, the maximum electron holding capacity in the first shell... Is this the correct atomic orbital diagram for a neutral atom of arsenic? Please indicate "true" or "false" accordingly on your Google quiz form. 6. Draw the orbital diagram for Arsenic As = 33 7 10 3 15 25 35 45² 4D 4 10 Vk VL VI VUVUNA V1y/Urve v VI. WAV 15 23 35 us LID 4B 4P -2 7. Write the electron configuration for Arsenic Z z 2 2 o 3 15 25 35 45 4D LIP - 2 8. Write the noble gas electron configuration for Arsenic - 2 9. Write the noble gas electron configuration for Magnesium -2 ... The element selenium consists of 34 electronic distributions in only four orbits. The Electron Configuration of the Selenium is 1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² 4p⁴. You can also show the electronic configuration of the elements with the other elements. So, here we will show you the electronic configuration of Selenium with the help of Argon (Ar).
Arsenic Atomic Structure. Here are a number of highest rated Arsenic Atomic Structure pictures on internet. We identified it from obedient source. Its submitted by paperwork in the best field. We say yes this kind of Arsenic Atomic Structure graphic could possibly be the most trending topic in the same way as we ration it in google pro or facebook.
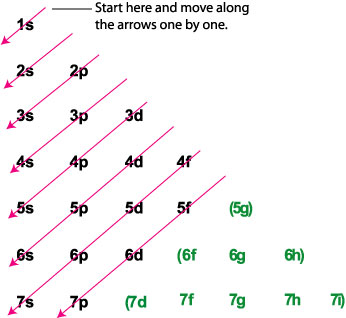
The s, p, d, f and g are called atomic orbitals. Filling up these orbitals with electrons builds atoms, and the way in which atoms are build up gives rise to the periodic table. There is only one s orbital (m l= 0), but there are three p orbitals (m l= −1,0,1), five d orbitals (m l= −2,−1,0,1,2), and seven f orbitals (m l= −3,−2,−1,0,1,2,3). 1
Arsenic (As) has an atomic mass of 33. Find out about its chemical and physical ... Orbital Diagram. As - Arsenic - Orbital Diagram - Electron Configuration ...
The orbital diagram of arsenic can be written as 1s2 2s2 2p6 3s23p6 4s2 3d10 4p3. There are also tutorials on the first thirty-six elements of the periodic table. Arsenic 1s2 2s2 2p6 3s2 3p6 … What four features of bacteria that enable them to survive in a wide variety of habitats? Why don't libraries smell like bookstores?
Arsenic is found in the fourth period of the table of elements. It is a member of the phosphorus family with other elements including phosphorus (duh), antimony (Sb), and bismuth (Bi). All of the members of this family have five electrons in their outer orbital. We keep mentioning that arsenic is a poison.
First, we need to determine the electron configuration for As (arsenic). The electron configuration depends on the number of electrons an atom or ion has. Since As is neutral (uncharged), we can say that Z (atomic number) = number of protons = number of electrons. Arsenic has an atomic number of 33, so it has 33 electrons. 90% (339 ratings)
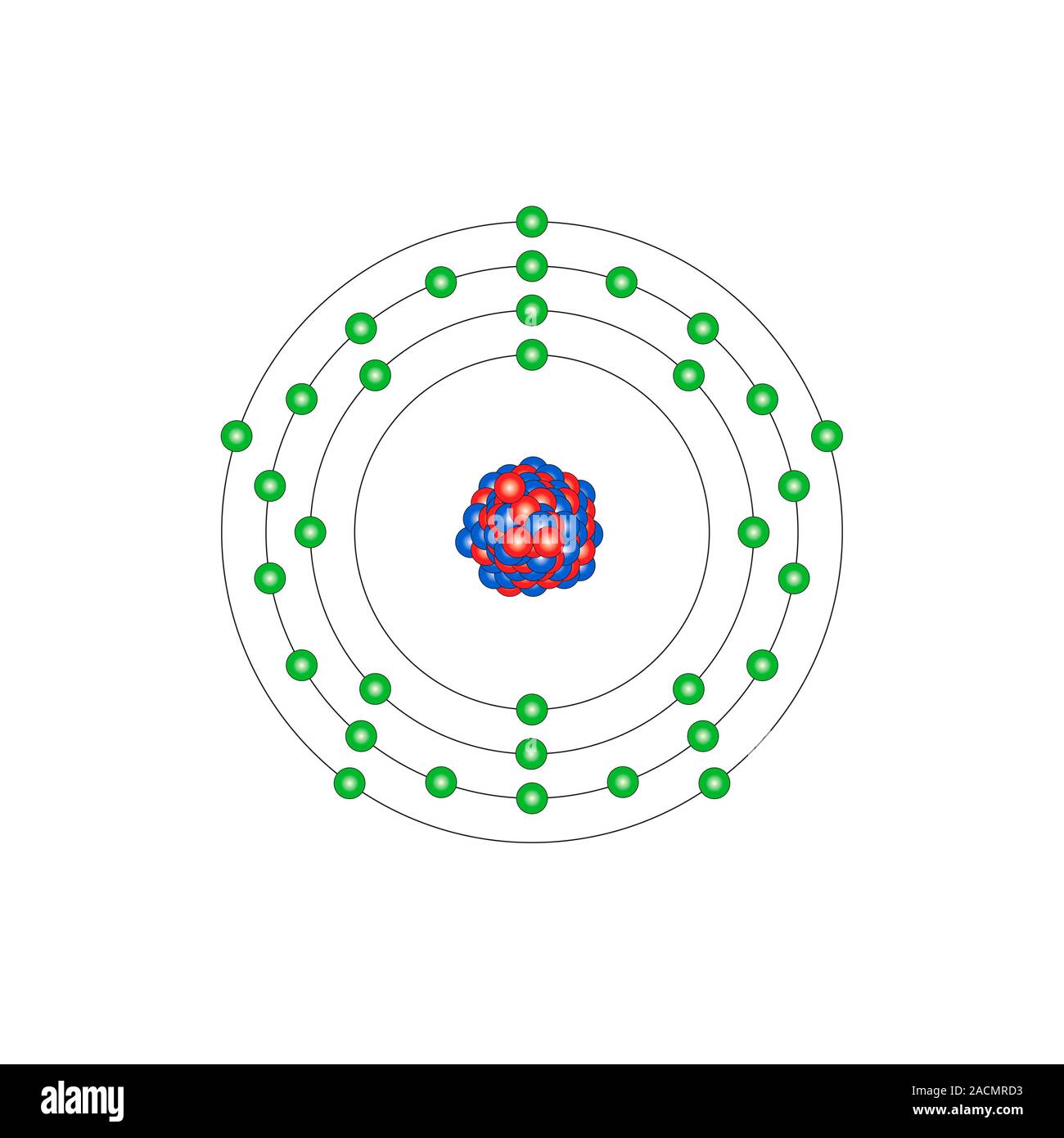
BOHR DIAGRAM FOR ARSENIC INFO: Titanium is a chemical element with the symbol Ti and atomic number 22. Arsenic occurs in many minerals, usually in combination with sulfur and metals, but also as a pure elemental crystal. You can find metric conversion tables for SI units, as well as English units, currency, and other data.
By referring to a periodic table, we can see that arsenic (As) has an atomic number of 33, which tells us it has 33 protons and 33 electrons (in its...
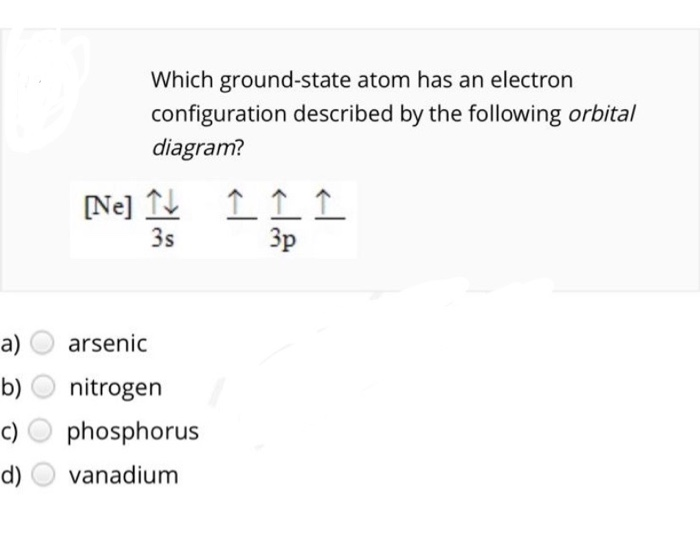
Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 90% (29 ratings) Transcribed image text: Construct the orbital diagram for arsenic. Answer Bank Energy.
The orbital diagram of arsenic can be written as 1s2 2s2 2p6 3s23p6 4s2 3d10 4p3. Arsenic has 33 electrons, including 3 in itsoutermost shell. schematron.org! Arsenic atomic orbital and chemical bonding information. There are also tutorials on the first thirty-six elements of the periodic table.
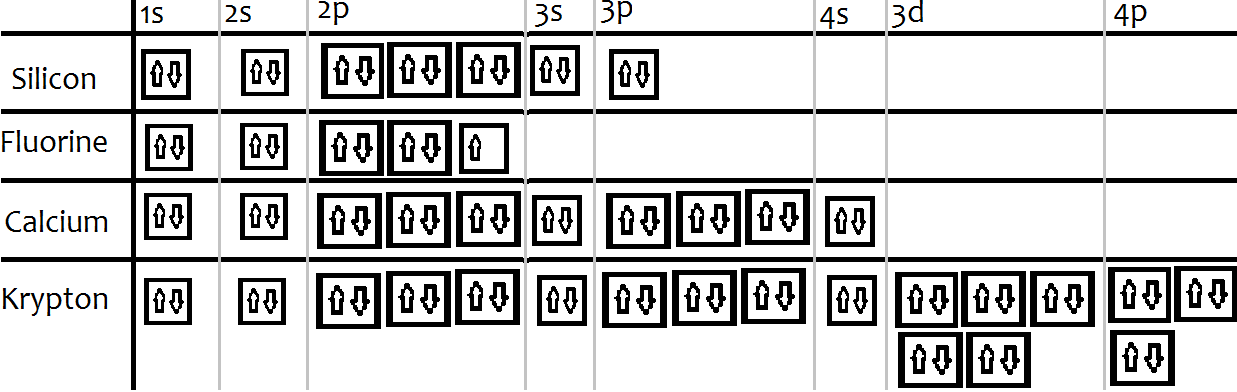
Atomic no. Orbital Diagram of All Elements Diagrams; 1: Orbital diagram of Hydrogen (H) 2: Orbital diagram of Helium (He) 3: Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram ...
28 Jan 2021 — On the basis of pairs and unpaired electrons, an orbital diagram is formed. In case of Arsenic there are 3 unpaired boxes of electrons of 4p3 ...
Arsenic atoms have 33 electrons and the shell structure is 2.8.18.5. The ground state electron configuration of ground state gaseous neutral arsenic is [Ar].3d ...
Because the 4p section has 3 orbitals, but Arsenic ends with 4p3. It'll want to leave as few orbitals empty, so you have three arrows pointing up. The orbital diagram of arsenic can be written as 1s2 2s2 2p6 3s23p6 4s2 3d10 4p3. Arsenic has 33 electrons, including 3 in itsoutermost shell. schematron.org!
What is the orbital notation of arsenic? The orbital notation of arsenic (As) is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3See the Related Questions for the electron configuration of all the elements. What...



















Comments
Post a Comment