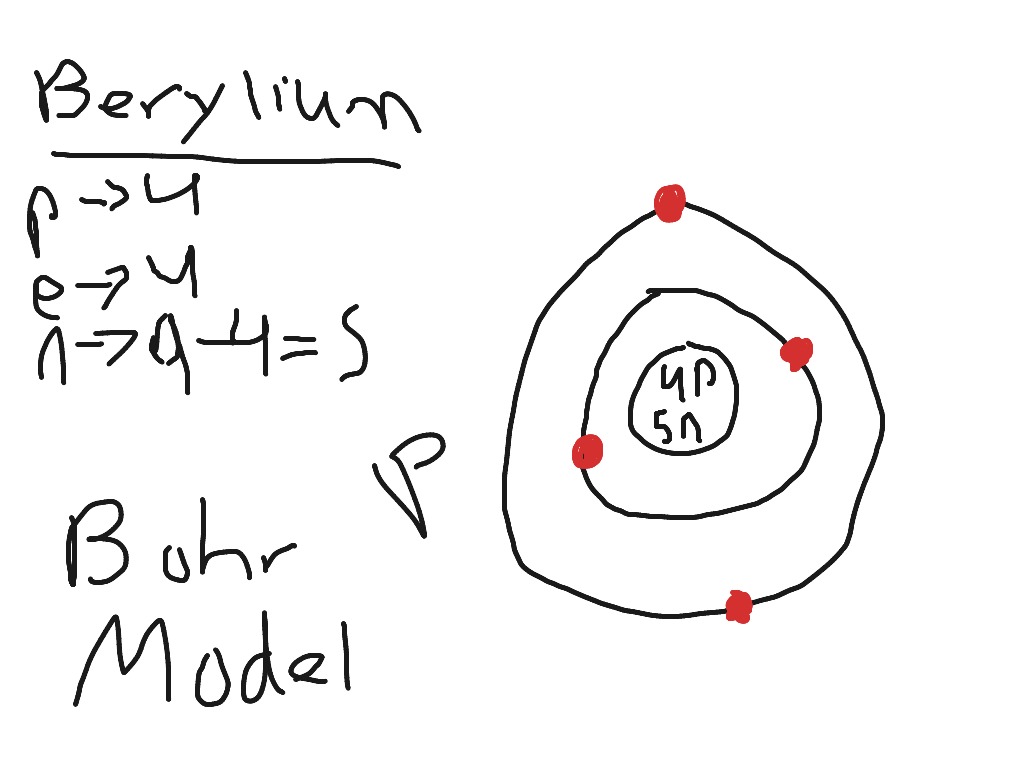
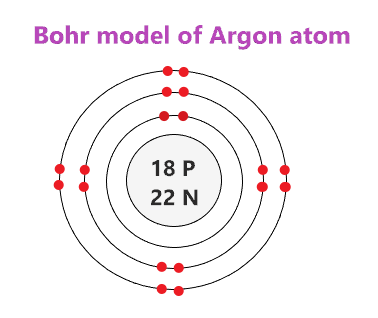
39 bohr diagram of oxygen
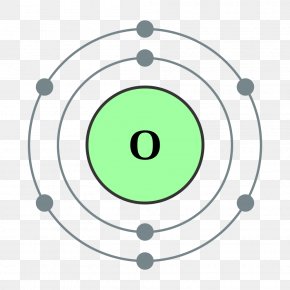
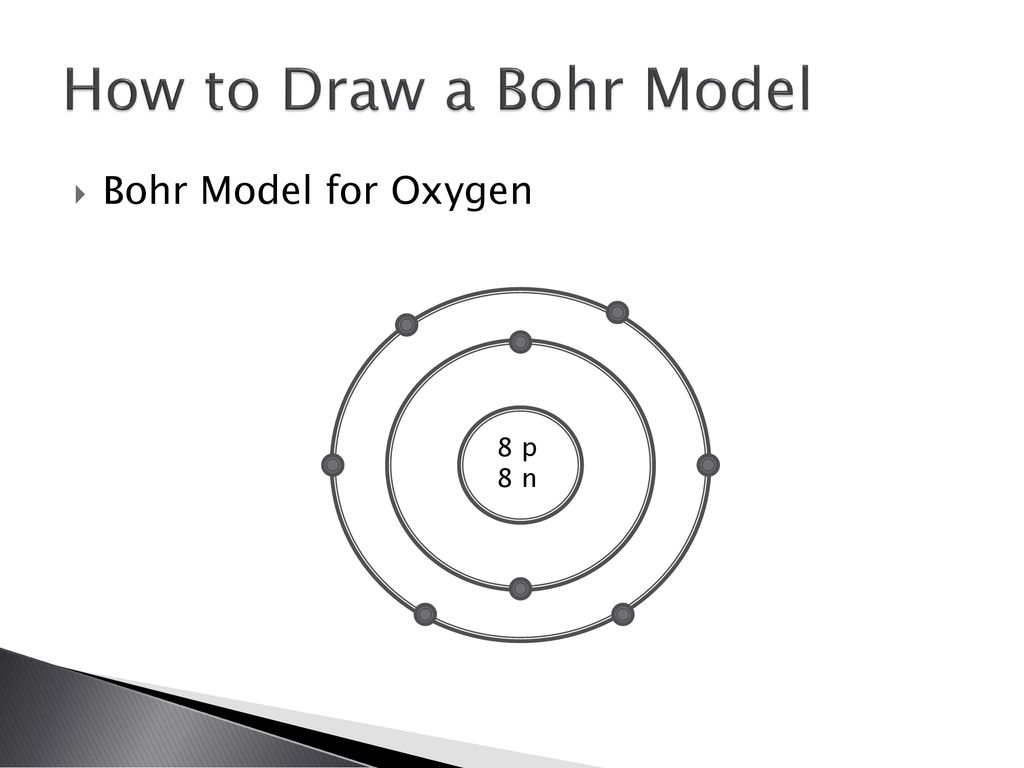
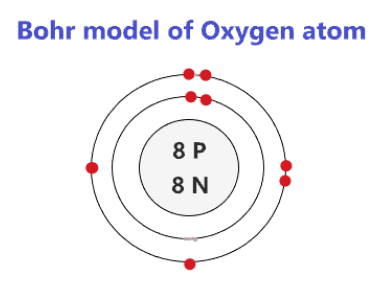
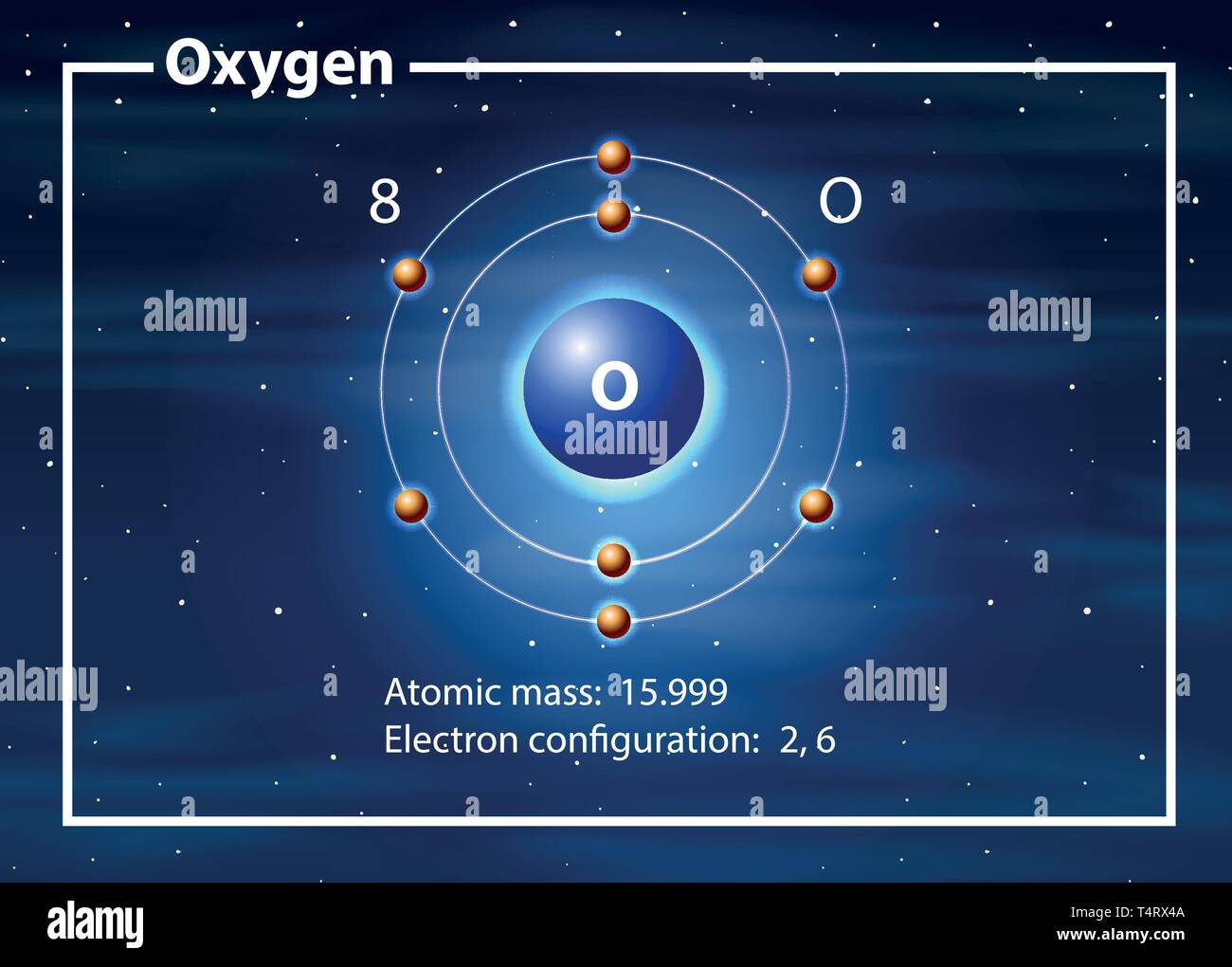
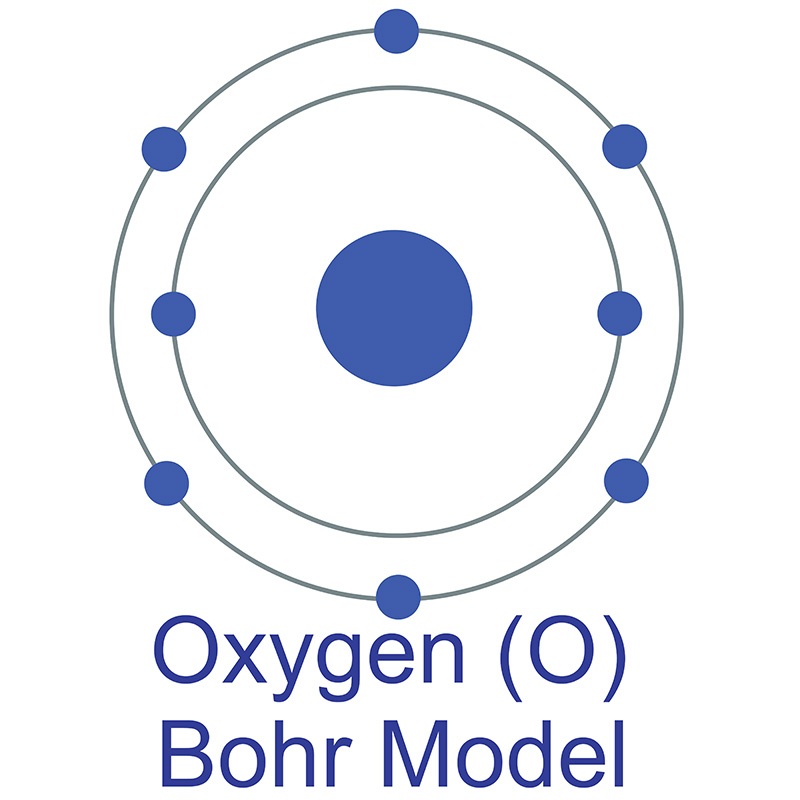
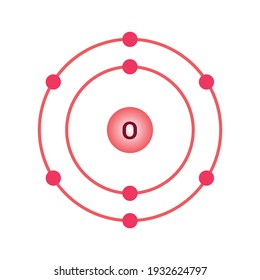
Atomic Structure — [Bohr Model of Oxygen], Number of Energy Levels: 2. First Energy Level: 2. Second Energy Level: 6. The Bohr model for oxygen shows eight protons and neutrons in the nucleus of the atom, with eight electrons orbiting the nucleus in two energy levels....
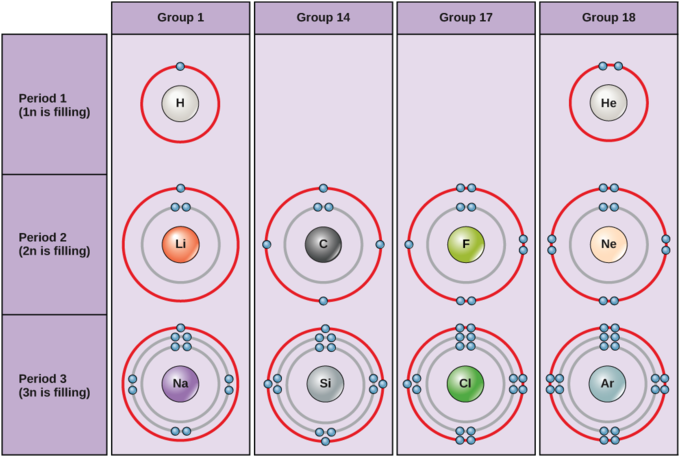
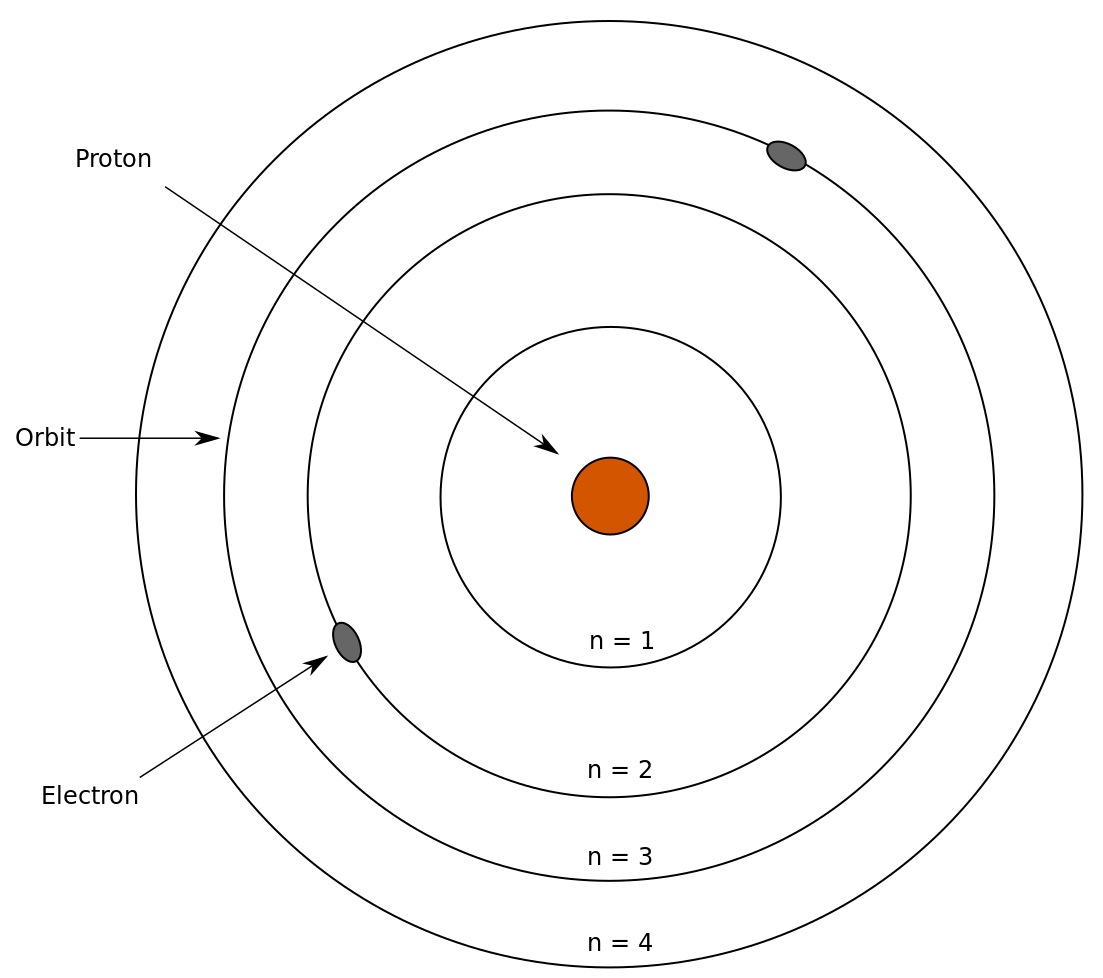
Bohr Models. Based on Niels Bohr's model of the atom, which said that electrons travel in set paths ("orbits") around the nucleus. Valence electrons (electrons in the outermost energy level) can be used to predict element . reactivity → how likely an element is to form a compound. with another element . How?
Bohr diagram of oxygen
A Bohr diagram is a simplified visual representation of an atom that was developed by Danish physicist Niels Bohr in 1913. The diagram depicts the atom as a positively charged nucleus surrounded by electrons that travel in circular orbits about the nucleus in discrete energy levels. This is the answer to the Stop and Jot from Lesson 1.4 for the Bohr Electron Configuration Drawing of the Oxygen Ion Bohr Diagrams 1) Check your work. 2) You should have 6 total electrons for Carbon. 3) Only two electrons can fit in the 1st shell. 4) The 2nd shell can hold up to 8 electrons. 5) The 3rd shell can hold 18, but the elements in the first few periods only use 8 electrons. 6p 6n. Bohr Diagrams Try the following elements one at a time: a) H b) He
Bohr diagram of oxygen. Bohr’s diagram of Oxygen has only two electron shells (K and L), the inner shell is K-shell and the outermost shell is L-shell. Hence, the electrons found in the L-shell of the Oxygen atom are its valence electrons because it is the outermost shell that also called the valence shell. Bohr-Rutherford Diagram. Number of protons in nucleus is 8. Number of neutrons in the nucleus is 8. Number of electrons in orbit around the nucleus is 8. Number of shells or orbits is 2. Like this: Bohr Diagrams • A Bohr diagram is a diagram that shows how many _____ are in each shell surrounding the nucleus. • Named in honour of _____, a Danish physicist who developed several models for showing the arrangement of electrons in atoms. • There are three main background questions to explore before we start drawing Bohr diagrams. answer choices. The bohr model diagram represents all the subatomic particles, while the lewis dot diagram only shows the symbol and the valence electrons. The lewis dot shows all the electrons and the bohr model only shows the electrons in the last shell. The lewis dot shows the number of neutrons and the bohr model only shows the electrons.
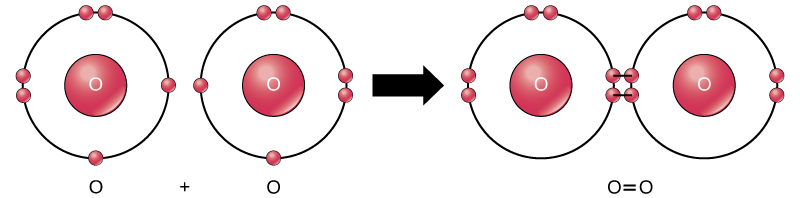
Oxygen (O) electron configuration with full orbital diagram. Oxygen (O) is the 8th element in the periodic table and the first element in group-16. The standard atomic mass of oxygen is 15.99903 and its symbol is 'O'. The period of oxygen is 2 and it is a p-block element. This article gives an idea about the electron configuration of oxygen ... We collected 40+ Bohr Model Drawing Oxygen paintings in our online museum of paintings - PaintingValley.com. ADVERTISEMENT. LIMITED OFFER: Get 10 free Shutterstock images - PICK10FREE. oxygen. model. bohr. diagram. atomic. rutherford. Drawing Bohr-Rutherford diagrams is super easy using the following steps: Find the number of protons, neutrons and electrons for the atom. The number of protons is the atomic number. …. Set up the diagram. To set up the diagram, you will need a circle in the middle. …. Add in orbitals and electrons. Bohr effect (medical or scientific explanation is down below) The Bohr effect explains the cell's oxygen release or why red blood cells unload oxygen in tissues, while carbon dioxide (CO2) is the key player in O2 transport due to vasodilation and the Bohr law. The Bohr law was first described in 1904 by the Danish physiologist Christian Bohr ...
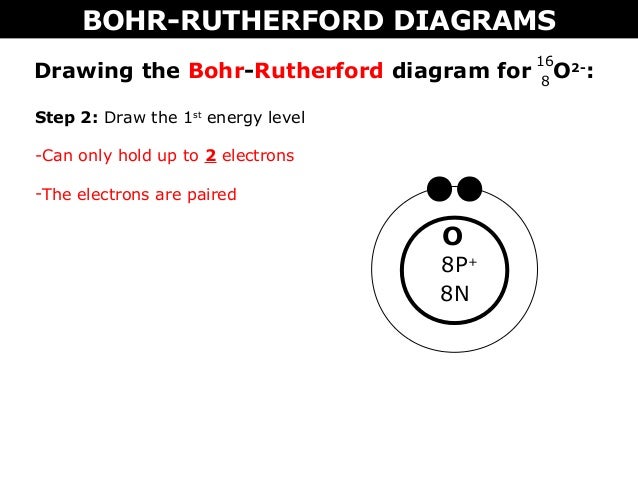
How to draw the Bohr-Rutherford Diagram for Oxygen. 2 electrons can go in the first shell, 8 in the second, 8 in the third, and so on... Bohr model of Beryllium (Be) 2, 2: 5: Bohr model of Boron (B) 2, 3: 6: Bohr model of Carbon (C) 2, 4: 7: Bohr model of Nitrogen (N) 2, 5: 8: Bohr model of Oxygen (O) 2, 6: 9: Bohr model of Fluorine (F) 2, 7: 10: Bohr model of Neon (Ne) 2, 8: 11: Bohr model of Sodium (Na) 2, 8, 1: 12: Bohr model of Magnesium (Mg) 2, 8, 2: 13: Bohr model of ... Aug 15, 2020 — Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are ... The bohr Rutherford diagram for oxygen has 8 protons and 8 neutrons. There are 2 electrons on the first orbital and six on the second. The bohr Rutherford diagram for oxygen has 8 protons and 8 ...
The bohr Rutherford diagram for oxygen has 8 protons and 8 neutrons. There are 2 electrons on the first orbital and six on the second. The bohr Rutherford diagram for oxygen h as 8 protons and 8 neutrons. Identification d'éléments à partir du diagramme de Bohr-Rutherford. Bohr - Rutherford Diagrams study guide by Mr_Vasiliou includes 26 ...
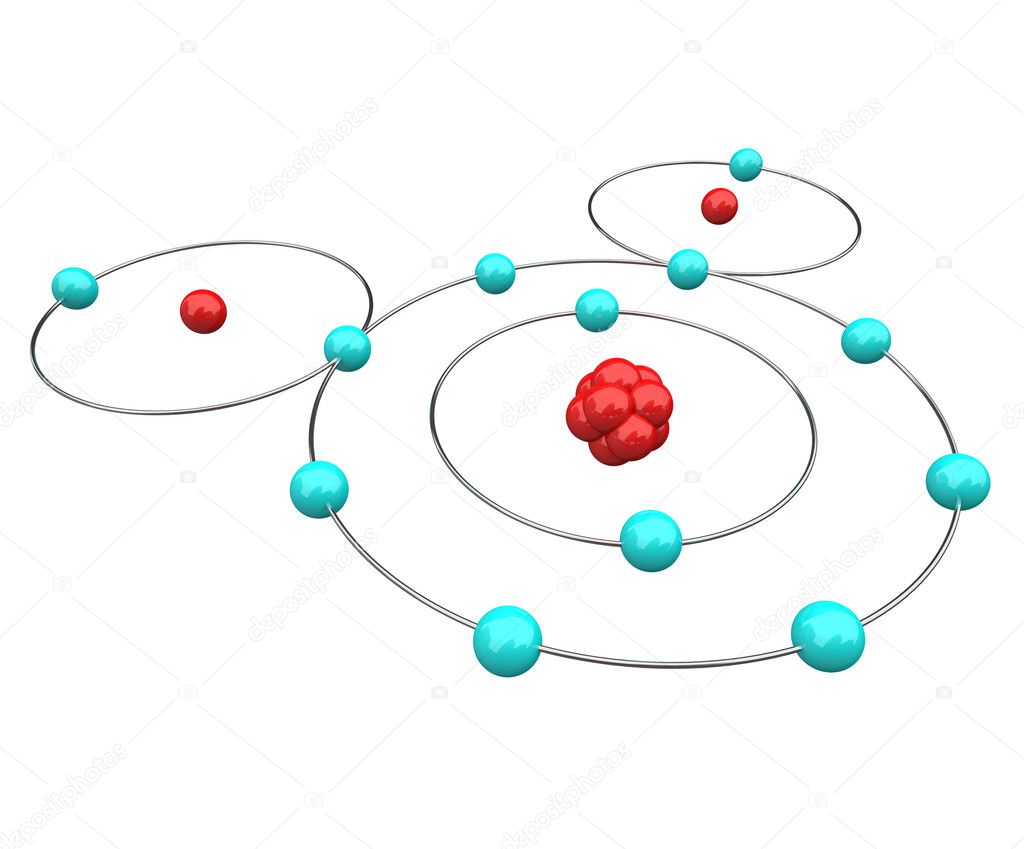
Bohr Diagram: The First Element. In order to make a Bohr diagram, you need to know the number of protons, neutrons, and electrons the element has. In this section, we'll show a sample Bohr diagram for hydrogen. H —Hydrogen. 1 proton. 1 electron. 0 neutrons
Jan 19, 2022 · Bohr Diagram For Oxygen. Here are a number of highest rated Bohr Diagram For Oxygen pictures upon internet. We identified it from reliable source. Its submitted by dealing out in the best field. We give a positive response this kind of Bohr Diagram For Oxygen graphic could possibly be the most trending topic like we part it in google gain or facebook.
Bohr Diagram Oxygen ... 960x720 0 0. Like JPG. Bohr Diagram For Oxy... 1466x1800 0 0. Like JPG. Bohr Model Oxygen Sc... 1024x768 0 0. Like JPG. Bohr Model Descripti... 600x400 0 0. Like JPG. Diagram Example Of A... 474x613 0 0. Like JPG. Diagram Of The Eye Q... 800x600 0 0. Like JPG. Diagram Of The Heart... 638x479 0 0. Like JPG. Do And Answer ...
The Bohr effect is a phenomenon first described in 1904 by the Danish physiologist Christian Bohr. Hemoglobin's oxygen binding affinity (see oxygen-haemoglobin dissociation curve) is inversely related both to acidity and to the concentration of carbon dioxide. That is, the Bohr effect refers to the shift in the oxygen dissociation curve caused by changes in the concentration of carbon ...
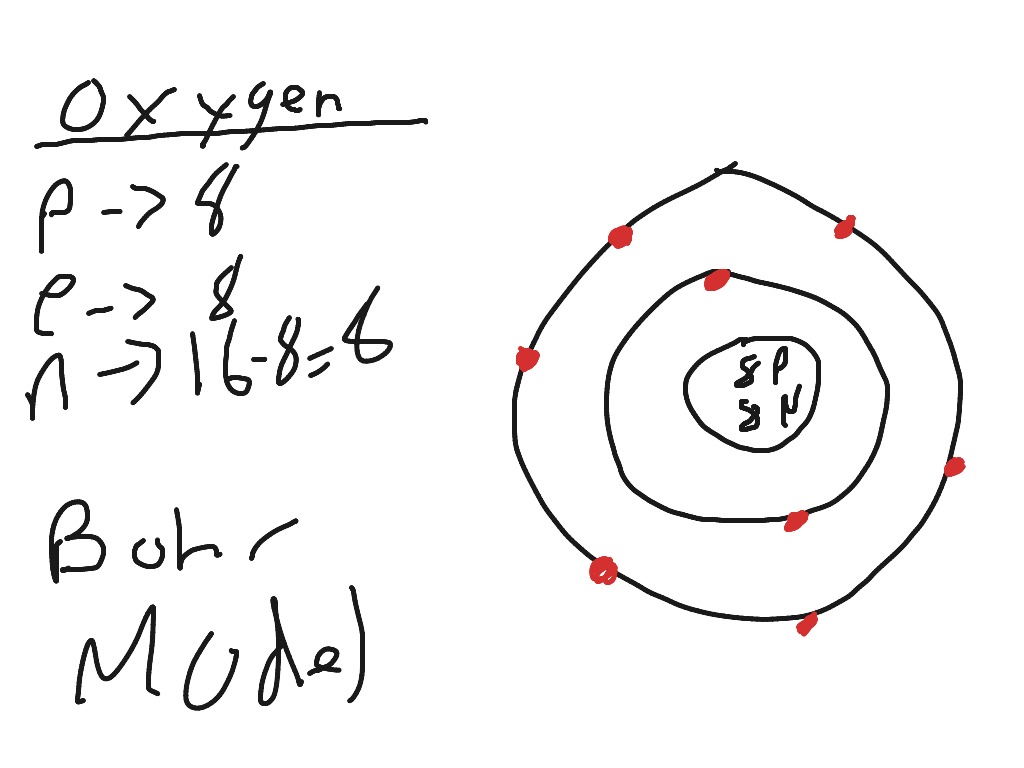
Its really hard to draw on the computer but if you see the number of protons and the number of neutrons are in the middle. Then the dots represent the number of electrons, (there are 8 electrons). So there will be a circle around the numbers and there will be 2 electrons on it, one on top one on the bottom, then draw another circle around that ...
The bohr Rutherford diagram for oxygen has 8 protons and 8 neutrons. There are 2 electrons on the first orbital and six on the second. The bohr Rutherford diagram for oxygen h as 8 protons and 8 neutrons. There are 2 electrons on the first orbital and six on the second. Facts Date of Discovery: Discoverer: Hans Christian Oersted Name Origin ...
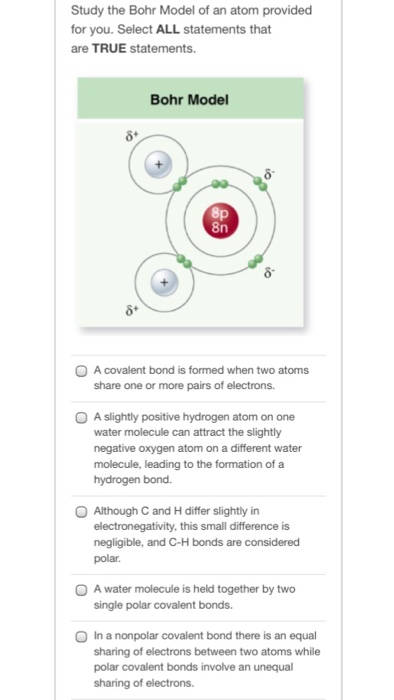
The following Bohr model diagram represents an oxygen atom. Examne the diagram, then answer the following questions a) Why is this not a stable electron arrangement? b) What would make this atom stable? c) use a different colored pen to adjust the dsagram so that it shows a stable electron arrangement.
Jun 23, 2020 · What is the Bohr diagram for oxygen? The Bohr model for oxygen shows eight protons and neutrons in the nucleus of the atom, with eight electrons orbiting the nucleus in two energy levels. Furthermore, what is a Bohr diagram? A Bohr diagram is a simplified visual representation of an atom that was developed by Danish physicist Niels Bohr in 1913.
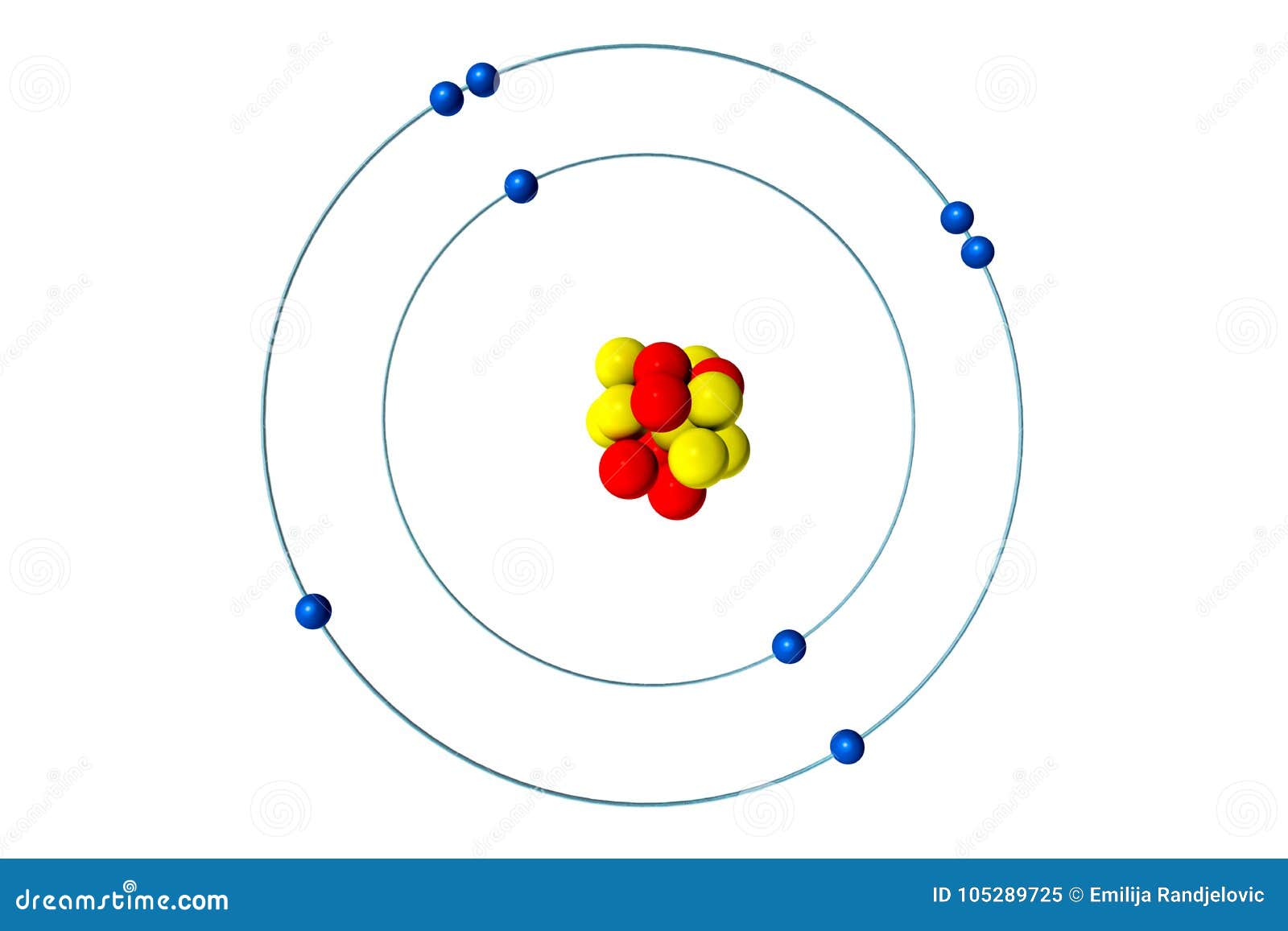
An example of a Bohr-Rutherford Diagram for oxygen is shown in Figure #2: Remember: Protons (p+) and neutrons (n0) are shown in the nucleus (centre).
Oxygen - Atomic Diagram Carbon Atom Bohr model with proton, neutron and electron. 3d illustration Bohr model of Nitrogen Atom with proton, neutron and electron.
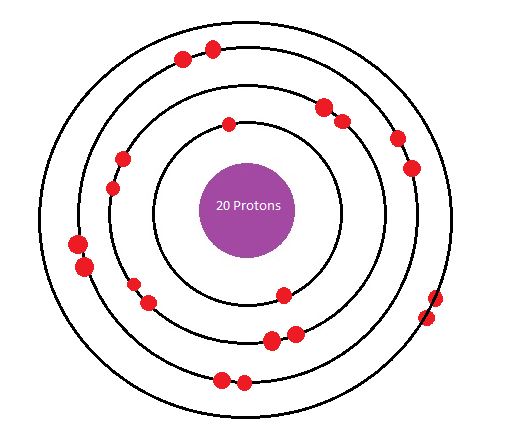
The Bohr Model of Calcium(Ca) has a nucleus that contains 20 neutrons and 20 protons. This nucleus is surrounded by four-electron shells named K-shell, L-shell, M-shell, and N-shell. The outermost shell in the Bohr diagram of Calcium contains only 2 electrons that also called valence electrons.
Bohr Diagrams 1) Check your work. 2) You should have 6 total electrons for Carbon. 3) Only two electrons can fit in the 1st shell. 4) The 2nd shell can hold up to 8 electrons. 5) The 3rd shell can hold 18, but the elements in the first few periods only use 8 electrons. 6p 6n. Bohr Diagrams Try the following elements one at a time: a) H b) He
This is the answer to the Stop and Jot from Lesson 1.4 for the Bohr Electron Configuration Drawing of the Oxygen Ion
A Bohr diagram is a simplified visual representation of an atom that was developed by Danish physicist Niels Bohr in 1913. The diagram depicts the atom as a positively charged nucleus surrounded by electrons that travel in circular orbits about the nucleus in discrete energy levels.



















Comments
Post a Comment